The ecological risk of pollutants
is closely related to their environmental behavior. A research team led by
Prof. Fan Qiaohui from the Northwest Institute of Eco-Environment and Resources
of the Chinese Academy of Sciences conducted an in-depth study on the
transformation process of As(III) on the surface of birnessite.
The researchers focused on semiconductor minerals with photoactive properties, revealing the important influence of such minerals on the chemical states of elements, subsequent transformation, migration, and environmental fate.
The study was published in npj Clean Water.
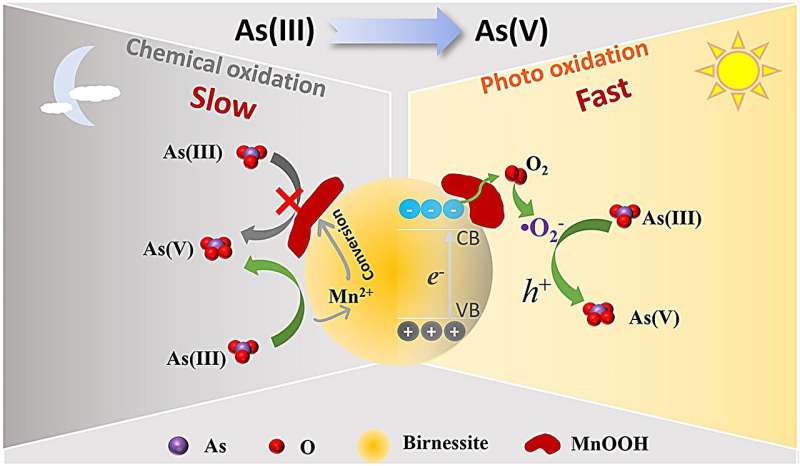
The researchers found that As(III) is chemically oxidized to As(V) by birnessite in the dark, while Mn(IV) is reduced to Mn(II). The reaction process also induces the formation of manganite (MnOOH). Since MnOOH covers the reaction sites, it inhibits further oxidation of As(III). After six hours of reaction in the dark, about 60% of As(III) is oxidized to As(V).
In addition, they also found that the photocatalytic oxidation of As(III) is significantly enhanced compared with chemical oxidation, and almost all of the As(III) is oxidized to As(V) by photo-generated holes and O2- radicals.
It is worth noting that, unlike chemical oxidation, MnOOH slightly affects the photocatalytic oxidation of As(III).
The researchers also used natural manganite ore as a photocatalyst and obtained the same results.
"This result shows that natural manganite ores have a significant effect on the environmental behavior of arsenic," said Prof. Fan, "this discovery provides important theoretical basis and experimental data for us to understand and control arsenic pollution."

 Previous page
Previous page Back to top
Back to top







