Clean drinking water is a basic
demand for our health and well-being. However, as the global population grows,
achieving this for all communities worldwide becomes more challenging.
Now, in a study published in Nature Communications, researchers from the HeKKSaGOn Alliance, which involved scientists from Kyoto University, Osaka University and Heidelberg University (Germany), have taken inspiration from a protein found in plants, creating a new way to remove harmful heavy-metal ions from water.
Although current water-purification methods are effective for the large volumes of water involved, they are generally not specific for heavy-metal ions. This means that they also remove ions that are not harmful, making these methods less efficient.
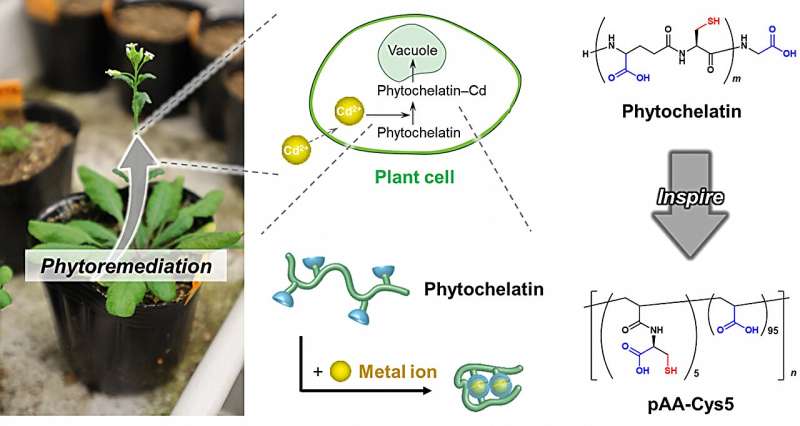
Phytochelatin, a highly conserved protein in plants, doesn't have this problem. Phytochelatin specifically binds to heavy-metal ions and then moves them to the cellular compartment where they are unable to cause harm.
The researchers wanted to know how phytochelatin achieves this. The team looked at the basic building blocks of phytochelatin to determine how it singles out and binds to certain ions, in this case, cadmium. "We identified two groups—carboxylate and thiolate—and synthesized a polymer containing these groups," explains lead author Masaki Nakahata from Osaka University.
High-throughput, integrative water treatment system based on pAA–Cys5–functionalized membrane and particles. Credit: Adapted from Nature Communications (2024). DOI: 10.1038/s41467-024-49869-8
By attaching the polymer to silica beads and cellulose membranes, the team was able to concentrate them into an ultra-small volume. The researchers then flowed contaminated water past the polymer, which removed cadmium ions from the sample very effectively, achieving an approved drinking level in one hour.
Having both carboxylate and thiolate groups present in the polymer structure was shown to be key to its success. Confining it to an ultra-small volume and creating a flow-through system dramatically pushed up the loading capacity of the polymer.
The system had high specificity for cadmium ions compared with metal ions essential to health, such as magnesium and calcium. This polymer can also show a comparably high affinity to mercury ions, indicating that it could be effective in removing other heavy metals.
"It is no surprise to us that plants gained such a highly sophisticated machinery during the evolution, because biology doesn't make nonsense," says Motomu Tanaka, senior author and a scientist at both Heidelberg University and Kyoto University. "However, we were excited to see that our plant-inspired polymer manages to even surpass what plant proteins can do."
The research team's achievement, synthesizing a polymer inspired by a plant protein to capture cadmium ions, is promising because the polymer is specific to toxic heavy-metal ions and works with a flow-through approach. These features are expected to improve the efficiency of water treatment—a win for clean water.

 Previous page
Previous page Back to top
Back to top







