A research team from Skoltech and
other scientific organizations conducted a study to determine which conditions
are the most suitable for storing graphene oxide—a promising material that can
be used for manufacturing composite materials, gas sensors, and in many other
fields.
The results show that the most optimal conditions for graphene oxide, when its properties will not change, are low temperatures and a lack of light. The paper was published in the Surfaces and Interfaces journal.
Graphene has unique properties and is widely used in electronics, energy storage, and optoelectronics. Obtaining pure graphene in large quantities is not easy and expensive, so it is often replaced with derivatives—for example, graphene oxide (GO). It has high electrical resistance, low thermal conductivity, and high solubility.
Graphene oxide has not yet been widely used in industry due to its chemical heterogeneity, structural disorders that occur due to the aggressive chemical environment during the synthesis, as well as natural aging of the material under environmental influence.
"The structure of graphene oxide manufactured chemically is very difficult to reproduce—it will always be different. And after a while, it begins to degrade, and the properties of the oxide itself change," said Dmitry Kvashnin, a study co-author, Doctor of Science in Physics and Mathematics, docent, Leading Research Scientist at the Emanuel Institute of Biochemical Physics of the Russian Academy of Sciences.
"If, after manufacturing, the material is sent to another place—another institute or country, it will come in a completely different state. And even when the test tubes are just there in the laboratory, the properties of the material also change. We decided to conduct a comprehensive study of the best conditions to store samples in."
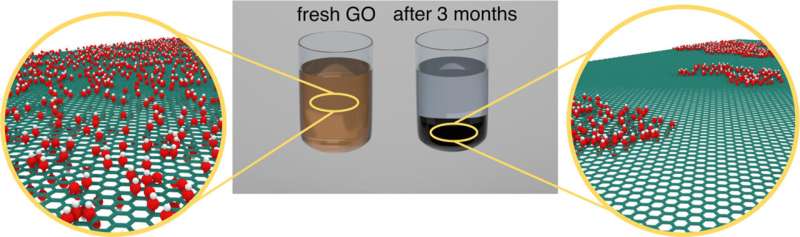
The team produced several samples of graphene oxide, identical in the chemical composition and production method, and placed them in different conditions: at room temperature and in the fridge, as well as in the light and without it.
"For 150 days, we observed changes in the properties of the samples. We looked at how the absorption spectra, X-ray photoelectron radiation spectra, hydrogen index, and viscosity of suspensions change. The comprehensive analysis of these characteristics allowed us to expand our understanding of the processes occurring on the surface of graphene oxide, leading to structural changes," said the first author of the study, Julia Bondareva, a research scientist at the Materials Center of Skoltech.
"We found out that graphene oxide is best stored in the cold and without exposure to light. In this case, there is no reduction, that is, oxygen-containing groups are not removed from the surface of graphene oxide, and it doesn't turn back into graphene. And at room temperature and in the light, it recovers faster. We can observe that even by the changing color of the solution, it goes darker."
"To find out what changes can occur in the structure of graphene oxide and why it precipitates over time, we used supercomputer atomistic modeling. Using quantum chemical calculations, we showed that in their most stable state, oxygen groups on the surface of graphene oxide tend to cluster," explained Nikita Orekhov, a co-author of the work, Deputy Head at the Laboratory of Computer Design of Materials at MIPT, Ph.D. in Physics and Mathematics.
"This differs from the bulk of the models used in the literature, which assume an even random distribution of oxygen. The clustering of oxygen groups that we showed, on the one hand, should lead to a change in optical spectra, and on the other, to the formation of pure graphene regions in those areas where oxygen 'migrated' from.
"Since graphene is an extremely hydrophobic material, such areas will tend to stick together to minimize contact with water. This is exactly what leads to precipitation observed in the experiment."
The results prove that special attention should be paid to the storage conditions of materials and to their properties at each stage of synthesis, the authors noted.

 Previous page
Previous page Back to top
Back to top







