The reason why water striders, water bugs, oarsman, diving bell spiders, and the like can walk on water or stay dry under it is that they have tiny hairs that prevent water actually coming into contact with their surface. These hairs produce spiky tops separated by valley-like gaps that, when submerged, creates a bubble between the material's surface and the tips of the spikes as the water clings to the top, while air, dissolved gases from the water, and water vapor are trapped beneath, which acts as a barrier between the surface and the water.
The researchers studied a variety of materials, some of which had the key surface roughness and some that did not, and compared how they performed in a series of experiments. Some of these involved different structures, or similar structures of different scales, or some that were tested in water where dissolved gases had been removed.
The results showed that some surfaces remained dry even after being submerged for four months, which was the length of the experiment. This included the samples in gasless liquids, which was surprising because it went against previous notions of how such structures worked. The original idea was that waterbugs and the like kept themselves dry by simple surface tension trapping air in the hairs around their bodies. However, this theory produced a number of problems, which are a matter of scale.
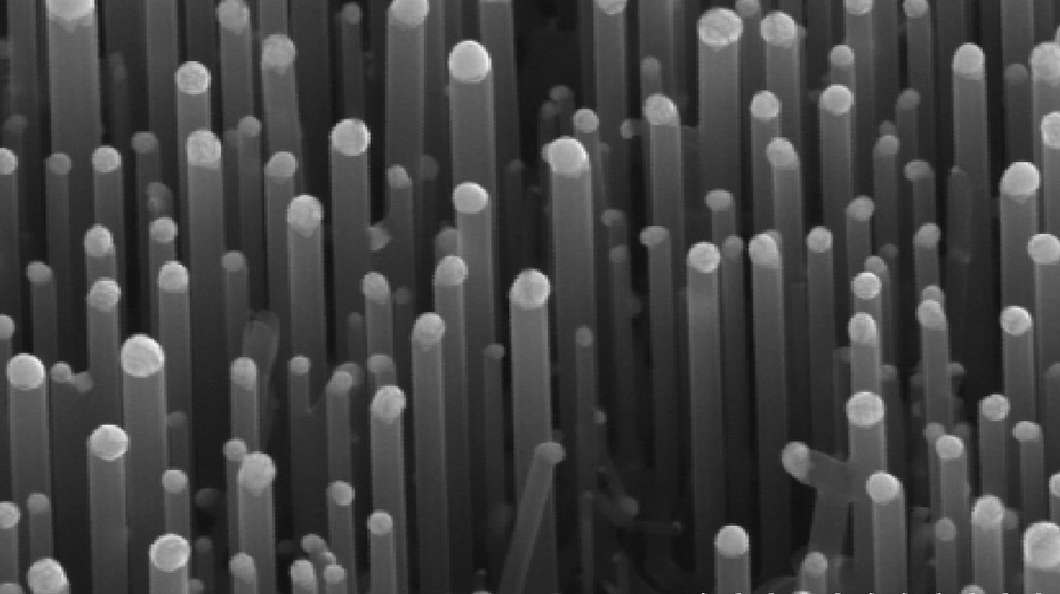
The team found that, conforming to the old theory, if the fibers or surface structure of the material was on a scale larger than a micron, the bubble was susceptible to a pair of weaknesses. First, the water vapor in the trapped air would condense and cause the bubble to shrink. Second, if the insect swam deeper, the increased pressure would squeeze the air. Either way, you end up with a wet bug.
The Northwestern experiments showed that water insects and spiders have hairs that create valleys that are less than a micron wide. This allows them to retain gas in a manner consistent with the team's models. Instead of being simply a matter of trapped air, the bubble is self-sustaining. The air inside is supplemented by water evaporating and producing water vapor. This offsets condensation and provides additional vapor, so the bubble doesn't squeeze down under pressure.
"The trick is to use rough surfaces of the right chemistry and size to promote vapor formation, which we can use to our advantage," says team leader Neelesh A. Patankar. "When the valleys are less than one micron wide, pockets of water vapor or gas accumulate in them by underwater evaporation or effervescence, just like a drop of water evaporates without having to boil it. These gas pockets deflect water, keeping the surface dry."
According to the team, these nanostructures have a wide variety of potential applications. Since they aren't unduly affected by pressure, they could be used on stealth coatings for submarines, in antifouling surfaces for ships to keep marine organisms from getting a grip on hulls, or as a pipe coating to reduce drag and speed up flow.

 Previous page
Previous page Back to top
Back to top







