Not content with using hybrid artificial photosynthesis to turn CO2 emissions into plastics and biofuel, researchers at the Lawrence Berkeley National Laboratory (Berkeley Lab) now claim to have produced an enhanced system that uses water and solar energy to generate hydrogen, which is in turn used to produce methane, the main element of natural gas, from carbon dioxide. Generating such gases from a renewable resource may one day help bolster, or even replace, fossil fuel resources extracted from dwindling sub-surface deposits.
Simply put, the process of photosynthesis turns light energy into chemical energy. In plants and certain types of algae, energy from incoming sunlight is used as the power source to synthesize simple carbohydrates from carbon dioxide and water. In the original Berkeley Lab hybrid system, a membrane arrangement of nanowires created from silicon and titanium oxide harvested solar energy and transported electrons to microbes where they used that energy to transform carbon dioxide into a range of chemical compounds.
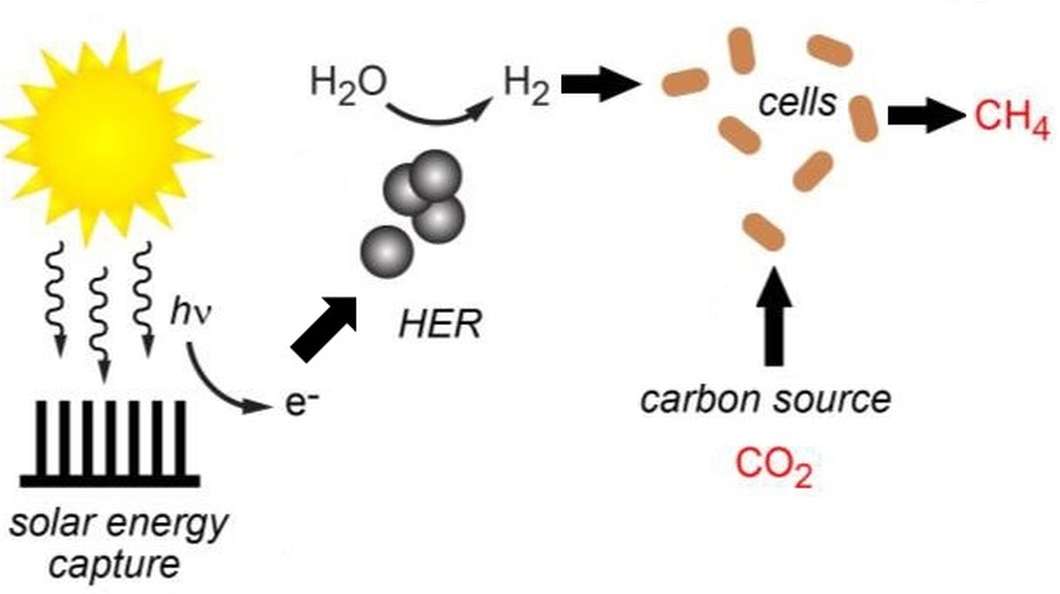
In the latest iteration of the artificial photosynthesis system, solar energy was captured via a similar membrane (but this time consisting of indium phosphide photocathodes and titanium dioxide photoanodes), which was employed to supply power for the splitting of water molecules into oxygen and hydrogen. The hydrogen was then conveyed to a collection of microbes that used it to convert carbon dioxide into methane. Hence the hybrid system collected light energy and produced both hydrogen and methane.
"This study represents another key breakthrough in solar-to-chemical energy conversion efficiency and artificial photosynthesis," said Professor Peidong Yang, a chemist with Berkeley Lab’s Materials Sciences Division. "By generating renewable hydrogen and feeding it to microbes for the production of methane, we can now expect an electrical-to-chemical efficiency of better than 50 percent and a solar-to-chemical energy conversion efficiency of 10-percent if our system is coupled with state-of-art solar panel and electrolyzer."
Though the fundamental concept in the two artificial photosynthesis experiments is largely similar, in the first tranche of work the researchers used an anaerobic bacterium, Sporomusa ovata, to transform carbon dioxide when fed with electrons. In the latest iteration, the scientists populated the membrane with Methanosarcina barkeri, which is an anaerobic archaeon (essentially a single-celled microorganism that has no cell nucleus or other membrane-bound organelles) that transforms carbon dioxide using hydrogen itself.
In this way, water is turned into hydrogen by a hydrogen evolution reaction (HER), where the HER is catalyzed by the addition of nickel sulfide nanoparticles that operate effectively under biologically compatible conditions.
"Using hydrogen as the energy carrier rather than electrons makes for a much more efficient process as molecular hydrogen, through its chemical bonds, has a much higher density for storing and transporting energy," said Associate Professor of chemistry at Berkeley Lab, and member of the research team, Michelle Chang.
"While we were inspired by the process of natural photosynthesis and continue to learn from it, by adding nanotechnology to help improve the efficiency of natural systems we are showing that sometimes we can do even better than nature," added Professor Yang.
Whilst this research is a multi-pronged approach to producing a range of gases and chemicals, it is also a method that brings living organisms into the mix. As such, even though purely electrical methods of solar hydrogen production are increasing in efficiency, and it is possible to use solar energy combined with cheap and abundant mineral elements to create hydrogen, the idea of generating a range of useful, energy-rich gases using just sunlight, water, CO2 and naturally-occurring microbes in a process scaled-up to commercial sizes holds a great deal of appeal in creating a truly environmentally-friendly and self-sufficient energy production system.
"We selected methane as an initial target owing to the ease of product separation, the potential for integration into existing infrastructures for the delivery and use of natural gas, and the fact that direct conversion of carbon dioxide to methane with synthetic catalysts has proven to be a formidable challenge," said Chris Chang, another professor of chemistry at Berkeley Lab and a member of the research team. "Since we still get the majority of our methane from natural gas, a fossil fuel, often from fracking, the ability to generate methane from a renewable hydrogen source is another important advance."

 Previous page
Previous page Back to top
Back to top







