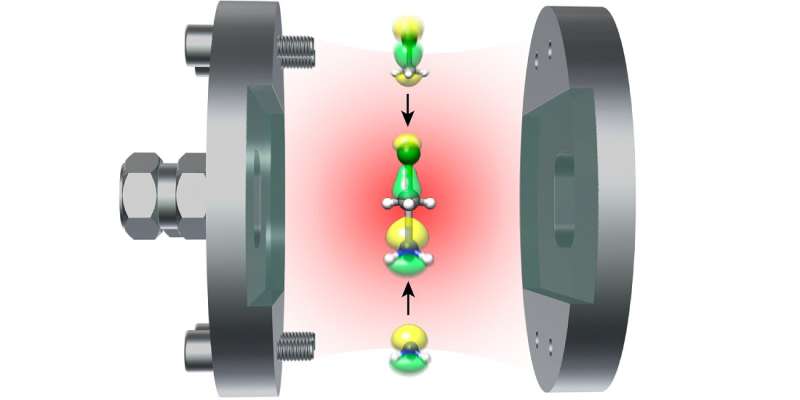
A research team from NTNU is
studying a topic called optical cavities and how the light trapped in them
interacts with atoms, molecules and other particles. The technology could prove
valuable for the development of energy-efficient chemical processes or drug
synthesis, for example.
The work of Professor Henrik Koch and Ph.D. candidates Rosario R. Riso, Tor S. Haugland and Marcus T. Lexander has shown startling results and is gaining attention.
"We've observed an effective method for describing molecules in optical cavities," says Professor Koch, who is employed both at NTNU's Department of Chemistry in the Faculty of Natural Sciences and the Scuola Normale Superiore di Pisa (SNS) in Italy.
Their results were recently published in Physical Review X and Nature Communications.
Optical cavities?
But what exactly are optical cavities? First of all, remember that on this scale, the world seems a little different than most of us are used to.
In quantum mechanics, particles and waves are indistinguishable because they have what's called a wave-particle duality, or a wave function.
Nor can we distinguish between particles and light in optical cavities, which have a molecule-light duality. This coupling creates new colors and properties in the molecules that can be utilized in chemical and physical processes.
Reflecting mirrors
Optical cavities can be created by using two mirrors that are extremely close together, typically nanometers apart from each other. To understand molecules requires looking at the environment they are in.
All atoms and molecules, like the oxygen in the Northern Lights, emit light because they interact with dim light that is always present in a vacuum, or "empty" space. The special quality in this case is that the light in an empty optical cavity is not the same as the light in the vacuum outside. Placing a molecule inside the cavity will change both the color and the intensity of the light emanating from the molecule.
"In an optical cavity made of reflecting mirrors, molecules can interact strongly with the quantum mechanic vacuum," says Koch.
The research team works exclusively with simulations, so it is important to collaborate with an experimental group that can test whether the team's theories are correct.
To this end, the research team is working with Professor John de Mello and Ph.D. candidate Enkui Lian from NTNU Nano to fabricate prototypes for use in research.
A common theory
Molecular orbital theory is an important theoretical tool in chemistry and is widely used in both inorganic and organic chemistry to understand chemical reactions.
"We've found the first consistent molecular orbital theory for quantum electrodynamics—that is, a molecular orbital theory for molecules in optical cavities," says Koch.
Using this theory, scientists can predict how molecules will react inside optical cavities, as well as what kinds of colors and properties the molecules will have.

 Previous page
Previous page Back to top
Back to top







