For the first time, an entirely
new class of super-reactive chemical compounds has been discovered under
atmospheric conditions. Researchers from the University of Copenhagen, in close
collaboration with international colleagues, have documented the formation of
so-called trioxides—an extremely oxidizing chemical compound that likely
affects both human health and our global climate.
Hydrogen peroxide is a commonly known chemical compound. All peroxides have two oxygen atoms attached to each other, making them highly reactive and often flammable and explosive. They are used for everything from whitening teeth and hair to cleaning wounds, and even as rocket fuel. But peroxides are also found in the atmosphere.
In recent years, there has been speculation as to whether trioxides—chemical compounds with three oxygen atoms attached to each other, and thereby even more reactive than the peroxides—are found in the atmosphere as well. But until now, it has never been unequivocally proven.
"This is what we have now accomplished," says Professor Henrik Grum Kjærgaard, at the University of Copenhagen's Department of Chemistry. Kjærgaard is the senior author of the study, just published in Science. "The type of compounds we discovered are unique in their structure. And, because they are extremely oxidizing, they most likely bring a host of effects that we have yet to uncover."
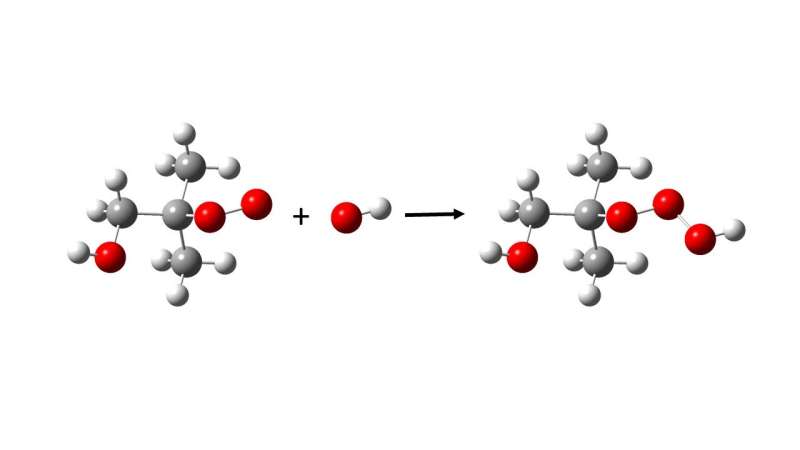
Hydrotrioxides (ROOOH), as they are known, are a completely new class of chemical compounds. Researchers at the University of Copenhagen (UCPH), together with colleagues at the Leibniz Institute for Tropospheric Research (TROPOS) and the California Institute of Technology (Caltech), have demonstrated that these compounds are formed under atmospheric conditions.
The researchers have also shown that hydrotrioxides are formed during the atmospheric decomposition of several known and widely emitted substances, including isoprene and dimethyl sulfide.
"It's quite significant that we can now show, through direct observation, that these compounds actually form in the atmosphere, that they are surprisingly stable and that they are formed from almost all chemical compounds. All speculation must now be put to rest," says Jing Chen, a Ph.D. student at the Department of Chemistry and second author of the study.
Hydrotrioxides are formed in a reaction between two types of radicals. The researchers expect that almost all chemical compounds will form hydrotrioxides in the atmosphere and estimate that their lifespans range from minutes to hours. This makes them stable enough to react with many other atmospheric compounds.
Presumably absorbed into aerosols
The research team also believes the trioxides are able to penetrate into tiny airborne particles, known as aerosols, which pose a health hazard and can lead to respiratory and cardiovascular diseases.
"They will most likely enter aerosols, where they will form new compounds with new effects. It is easy to imagine that new substances are formed in the aerosols that are harmful if inhaled. But further investigation is required to address these potential health effects," says Henrik Grum Kjærgaard.
While aerosols also have an impact on climate, they are one of the things that are most difficult to describe in climate models. And according to the researchers, there is a high probability that hydrotrioxides impact how many aerosols are produced.
"As sunlight is both reflected and absorbed by aerosols, this affects the Earth's heat balance—that is, the ratio of sunlight that Earth absorbs and sends back into space. When aerosols absorb substances, they grow and contribute to cloud formation, which affects Earth's climate as well," says co-author and Ph.D. student, Eva R. Kjærgaard.
Compound's effect needs to be studied further
The researchers hope that the discovery of hydrotrioxides will help us learn more about the effects of the chemicals we emit.
"Most human activity leads to emission of chemical substances into the atmosphere. So, knowledge of the reactions that determine atmospheric chemistry is important if we are to be able to predict how our actions will affect the atmosphere in the future," says co-author and postdoc, Kristan H. Møller.
However, neither he nor Henrik Grum Kjærgaard are worried about the new discovery: "These compounds have always been around—we just didn't know about them. But the fact that we now have evidence that the compounds are formed and live for a certain amount of time means that it is possible to study their effect in a more targeted way and respond if they turn out to be dangerous," says Henrik Grum Kjærgaard.
"The discovery suggests that there could be plenty of other things in the air that we don't yet know about. Indeed, the air surrounding us is a huge tangle of complex chemical reactions. As researchers, we need to keep an open mind if we want to get better at finding solutions," concludes Jing Chen.

 Previous page
Previous page Back to top
Back to top







