Electrochemical devices such as fuel cells are becoming indispensable for new power generation technologies because they can efficiently produce renewable energy. Ceramic proton conductors can be used in many applications, including protonic ceramic fuel cells (PCFCs), hydrogen pumps, sensors, and separation membranes.
In particular, PCFCs based on ceramic proton conductors are promising, because they can work at lower temperatures compared with conventional solid oxide fuel cells (SOFCs), thanks to the higher conductivity of protons at low temperatures.However, conventional ceramic proton conductors face one problem: In order to exhibit adequate proton conductivity, they need to have oxygen vacancies that enable water incorporation. In most cases, the vacancies are created via chemical substitution, which is often a difficult process.
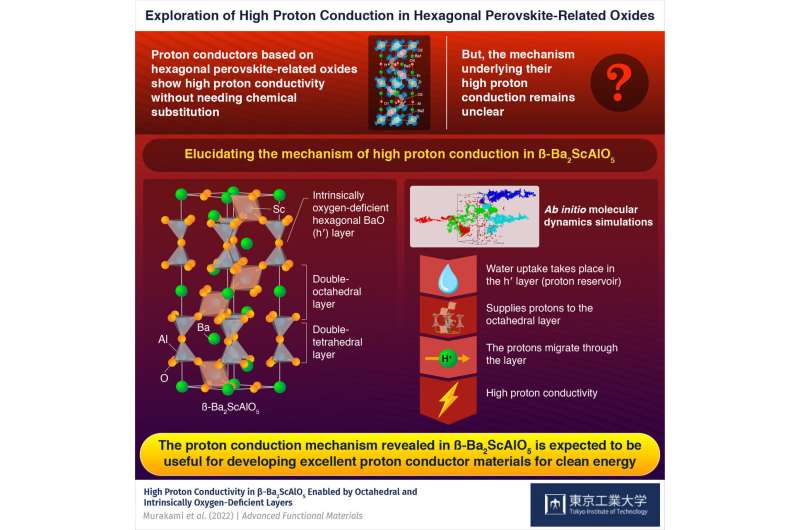
Now, a group of researchers led by Professor Masatomo Yashima of Tokyo Tech's Department of Chemistry has explored the proton-conducting hexagonal perovskite-related oxides instead. The crystal structure of these oxides contains layers that are intrinsically oxygen-deficient, which enables high proton conductivity without chemical substitution. However, their conduction mechanism remains unclear.
To shed light on this, the research group led by Professor Yashima recently analyzed and compared three types of oxides: β-Ba2ScAlO5, α-Ba2Sc0.83Al1.17O5, and BaAl2O4. The oxide-deficient layers of all these three oxides have different stacking patterns. The team found that while β-Ba2ScAlO5 showed high proton conductivity, the structurally related α-Ba2Sc0.83Al1.17O5 and BaAl2O4 had much lower conductivities.
The group's results were published in Advanced Functional Materials.
Prof. Yashima briefly explains the crystal structure of β-Ba2ScAlO5: "It consists of double-octahedral layers separated by double-tetrahedral layers. The latter have hexagonal BaO (h') layers that are intrinsically oxygen deficient. Their roles in proton conduction have been explored through various methods."
First, the researchers found that the ion conductivity of β-Ba2ScAlO5 was many-fold (ex. 31 times) higher in wet conditions than in dry air. This was due to the material absorbing water from the wet air, leading to higher proton concentrations and conductivity. The proton conductivity was found to be as high as 10−3 S cm‒1 above 300 °C—a value comparable to that of conventional, chemically substituted conductors.
Bond valence-based energy and density functional theory calculations revealed that this water uptake occurs in the h' layers of the oxide. Additionally, ab initio molecular dynamics simulations showed that these layers act as reservoirs, supplying protons that migrate via long-range diffusion in the double-octahedral layers. This phenomenon leads to the high proton conductivity of β-Ba2ScAlO5.
In contrast, BaAl2O4 displayed much lower conduction due to less water uptake, low proton mobility, and the absence of octahedral layers. These observations further validate the significant roles of both octahedral and oxygen-deficient layers in proton conduction.
"The study is a great example of tackling complex research problems through collaboration and showcases ANSTO capabilities and expertise in neutron scattering and scientific computing. The Echidna diffractometer at the OPAL reactor was used to elucidate crystal structure and molecular dynamics simulations also performed at ANSTO shed light on the proton conductivity mechanism," said Prof. Max Avdeev of ANSTO.
Prof. Yashima discusses the future potential of the team's work: "Our results offer a strategy for designing superior hexagonal perovskite-related oxides with octahedral layers and intrinsically oxygen-deficient layers. Combining these layers with different roles can produce superior proton conductors for renewable energy production and storage devices."

 Previous page
Previous page Back to top
Back to top







