Stimuli-responsive materials,
whose physical properties change in response to external stimuli such as light
and heat, are being widely studied as next-generation functional materials.
Photo-responsive materials in particular are attracting much attention because
their physicochemical properties can be modulated remotely without any physical
contact.
As one of the photo-responsive materials, photomechanical molecular crystals, which consist of molecules that undergo photochemical reactions upon light irradiation, are being intensively investigated because of their unique photoresponsive behaviors.
Unlike in solutions where molecules exist independently, the molecules in crystals are arranged densely and regularly, so the unique photoreaction dynamics in crystals must be considered. However, previous studies on photoreactive molecular crystal materials have usually assumed that photoreactions in crystals proceed like those in solutions and follow classical photoreaction kinetics. Understanding of physicochemical property changes based on the photochemical reaction kinetics in crystals has therefore been an issue for further development of this research field.
A research group led by Kohei Morimoto, a third-year doctoral student at the Osaka City University Graduate School of Engineering, and Dr. Daichi Kitagawa and Professor Seiya Kobatake at the Osaka Metropolitan University Graduate School of Engineering has discovered that photoreactions in 2,5-distyrylpyrazine (DSP) crystals propagate from each of the crystal's edges to its center. The research results were published online in Angewandte Chemie International Edition on November 3, 2022.
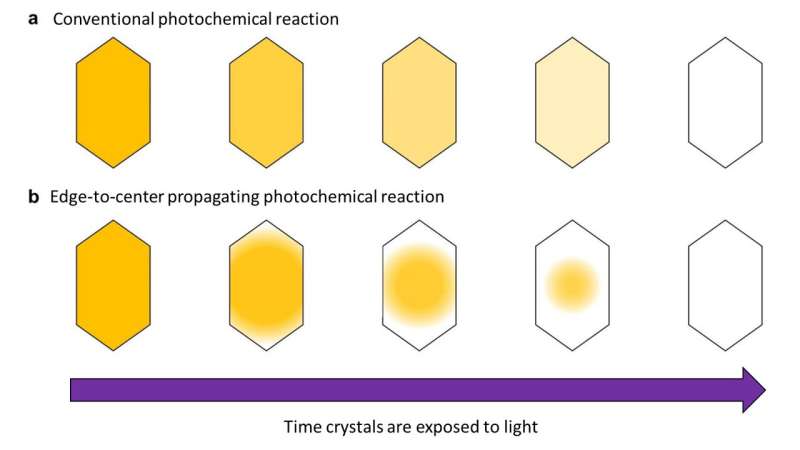
Normally, when light shines evenly on a crystal, the color change caused by the photoreaction also proceeds evenly. However, the research group found that in DSP crystals, the color change started at the crystal's edge and proceeded to its center, propagating the photoreaction like a wave.
The research group determined that this edge-to-center propagation of the color change happens for two reasons: a surface effect, which makes reactivity extremely high at the edges of the crystal, and a cooperative effect, which also elevates reactivity for molecules whose neighbors have already changed color.
"This phenomenon, which deviates from the conventional conception of photochemistry, is hugely significant for how we understand the basic fundamental science of photoreactions," said Dr. Kitagawa.
"In the future, we would like to clarify the conditions necessary for this unique photoreaction to occur and explore how they can be used to create new functional materials that take advantage of this phenomenon."

 Previous page
Previous page Back to top
Back to top







