Dr. Michal Mazur and his
colleagues from the Faculty of Science, Charles University in Prague study
catalysts that are based on metal nanoparticles stabilized at zeolites.
Recently, they have prepared a new type of zeolitic catalyst. Their results
have been published in the journal Angewandte Chemie.
Many chemical processes, such as oxidation, hydrogenation, dehydrogenation, and reforming reactions require the use of heterogeneous catalysts based on transition metals. The price of some of these metals, such as rhodium or platinum, is high; thus, the efficiency of their utilization is a key factor for industrial use. One of the possible solutions is to prepare them in the form of nanoparticles, which allows the exposure and effective use of a larger fraction of metal atoms.
"This situation has many equivalents in regular life. Let's say you want to open a business based on selling coffee in Prague. It is much better to open many small coffee shops in different parts of the city, than only one big shop in the city center. This allows your business to be more accessible to customers, thus efficient," says Dr. Mazur in describing his strategy.
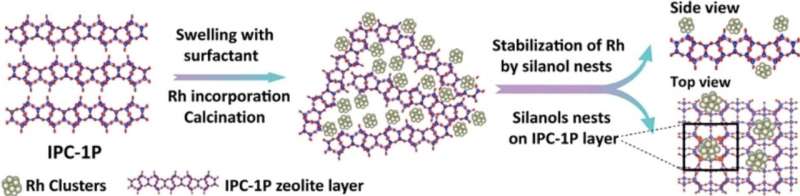
Similarly, it is better to prepare a catalyst with a lot of small, well-distributed nanoparticles, than a few big pieces of metal, where only the surface is active and inner atoms are not accessible for reactants. Due to this fact, a lot of researchers' effort is dedicated to stabilizing small metal nanoparticles at the supports. One of the possible and often-used supports is zeolites. They have several appropriate features for the encapsulation of metal, including rigid frameworks, physical and chemical stability, high surface areas, ordered microporous channels, and tunable acid sites. Overall, they show a lot of additional functionalities as potential supports for metal nanoparticle catalysts.
"In our new work, we used layered zeolite and its features to stabilize rhodium nanoparticles at the surface of these layers. We found that the specific geometry and location of functional groups (silanols) at the layer surface can make nanoparticles stable, even at high temperatures or when exposed to harsh conditions, like oxidation-reduction cycles or catalyst regeneration," explains Dr. Mazur of the research findings.
"We showed that the resulting material is an active hydrogenation catalyst with huge potential to be selective towards bulky molecules. Our findings were proved not only by advanced experimental techniques, such as in-situ transmission electron microscopy but also confirmed by theoretical DFT calculations. This finding showed new insight into the design of catalysts and opened new pathways in zeolite chemistry, which is why we will continue the research in this area," he concludes.

 Previous page
Previous page Back to top
Back to top







