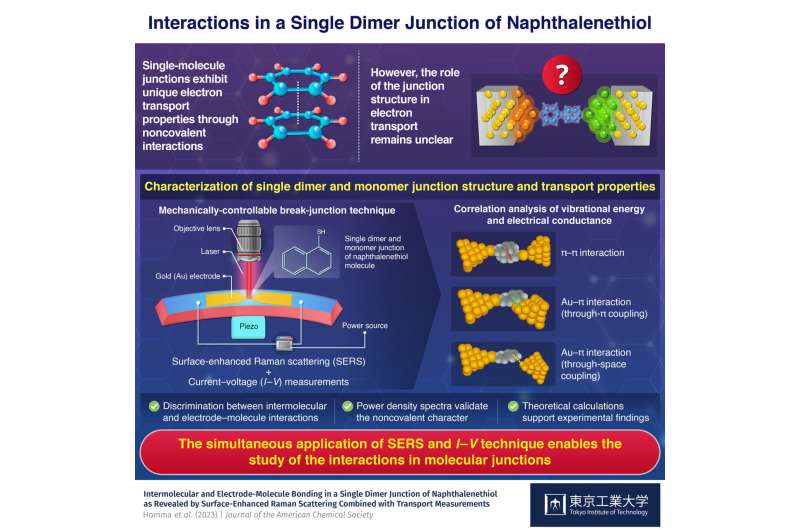
The structure of a molecular
junction with noncovalent interaction plays a key role in electron transport,
reveals a recent study conducted by researchers at Tokyo Tech. Through
simultaneous surface-enhanced Raman scattering and current–voltage measurements,
they found that a single dimer junction of naphthalenethiol molecule shows
three different bondings, namely π–π intermolecular and through-π and
through-space molecule–electrode interactions.
The π–π interaction is a type of noncovalent interaction that occurs when the electron clouds in the π orbitals of aromatic rings or π-conjugated molecular systems overlap. This interaction enables an efficient movement of electrons between the molecules, offering the potential to design materials with unique electronic properties.
The structure of the junctions formed by these molecules plays a decisive role in electron transport. However, insufficient structural information on these junctions has made it challenging to establish a clear relationship between the structure and electron transport properties.
To address this knowledge gap, a group of researchers from Japan, led by Assistant Professor Satoshi Kaneko and Associate Professor Tomoaki Nishino from Tokyo Institute of Technology (Tokyo Tech), has recently fabricated a single dimer and monomer junction of naphthalenethiol (NT) molecule and conducted a detailed examination of their structure and electron transport properties using combined optical and electrical measurements. Their study was published recently in the Journal of the American Chemical Society.
The researchers fabricated the junction by first depositing a gold electrode on a phosphor bronze plate coated with a polyimide layer. Next, they selectively removed the polyimide material beneath the central region of the gold electrode, forming a free-standing structure. Finally, they added ethanol solution containing NT dropwise on to the substrate, resulting in the formation of a single layer of NT molecules linking the gold electrodes.
Having fabricated the junction, the researchers then conducted simultaneous in situ surface-enhanced Raman scattering (SERS) and current–voltage measurements (I–V) by employing the mechanically-controllable break-junction technique. "This was followed by a correlation analysis of the measured vibrational energy and electrical conductance values, enabling the identification of the intermolecular and molecule–electrode interactions and transport properties in the NT junction," explains Dr. Kaneko.
The current–voltage measurements revealed distinct high-conductivity and low-conductivity states. While a high-conductance state originated from an NT–monomer junction, where the molecule interacts directly with gold electrodes through direct π-bonding, the low-conductance state emerged due to an NT dimer formed by intermolecular π–π interaction.
However, considering vibrational energy alongside conductance confirmed three distinct structures at the junction, corresponding to a high-conductance state and two low-conductance states, respectively. When the naphthalene ring—in both dimer and monomer configurations—directly interacted with the gold electrodes through π coupling, highly conductive junctions were formed. Conversely, weak interactions between the naphthalene ring and the gold electrode through space coupling resulted in weakly conductive junctions.
"The simultaneous application of SERS and I-V technique could discriminate the various noncovalent interactions in the NT molecular junction, shedding light on its electron transport properties. In addition, the noncovalent character was also revealed by the power density spectra," says Dr. Nishino.
The present findings thus provide important insights into π–π interactions that could pave the way for utilizing aromatic molecules in the design of future electronic devices and technologies.

 Previous page
Previous page Back to top
Back to top







