Researchers
who are working to find alternatives to lithium-ion batteries have turned their
attention to potassium-ion batteries. Potassium is an abundant resource, and
the technology functions in much the same way as lithium-ion batteries, but
these batteries have not been developed at a large scale because the ionic
radius causes problems in energy storage and substandard electrochemical
performance.
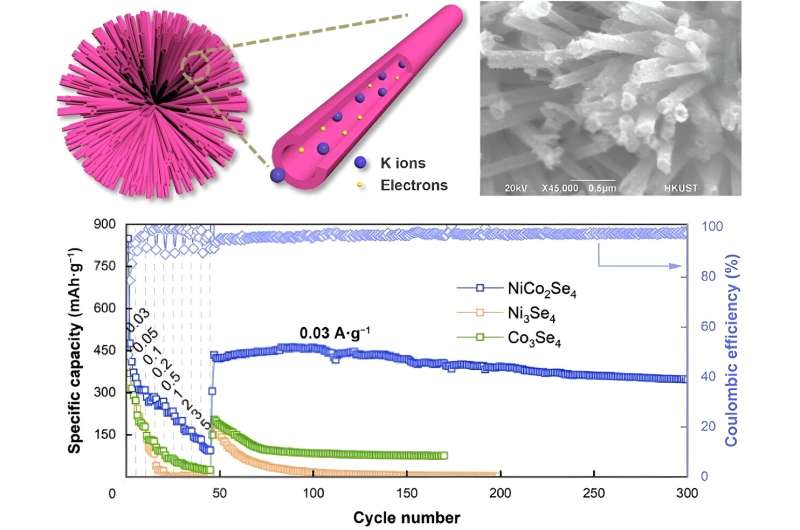
To solve this problem, researchers are considering NiCo2Se4, a bimetallic selenide, to create sphere-shaped electrodes. The spheres are constructed with NiCo2Se4 nanotubes, which improve the electrochemical reactivity for faster transfer and storage of potassium ions.
The research was presented in a paper published in Energy Materials and Devices on 14 September.
"Bimetallic selenides combine the ameliorating features of two metals, which synergize by showing rich redox reaction sites and high electrochemical activities. One bimetallic selenide, NiCo2Se4, was previously studied for sodium storage, supercapacitors, and electrocatalysts and presents considerable potential for potassium ion storage.
"By synthesizing NiCo2Se4 using a two-step hydrothermal process, a nanotube structure with flower-like clusters develops, creating convenient channels for potassium ion/electron transfer," said Mingyue Wang, a researcher at the Engineering Research Center of Energy Storage Materials and Devices at Xi'an Jiaotong University in Xi'an, China.
Initially, Ni-Co precursor spheres with solid nanoneedles are prepared. These spheres have a well-defined crystalline structure that is then exposed to selenide during a process called selenization. This process introduces selenium to the Ni-Co precursor, developing the NiCo2Se4 nanotube shell.
The hollow tubes form because of a phenomenon called the Kirkendall effect, which is when two metals move because of the difference in the diffusion rates of their atoms. These nanotubes are around 35 nanometers wide, giving enough space for the potassium ions and electrons to transfer.
Through a variety of tests and analysis, the researchers were able to confirm how well the NiCo2Se4 anodes could move and store potassium ions and electrons. They found that NiCo2Se4 has more active sites than other electrode materials, had uniformly distributed elements, and outperformed other electrodes that were tested during research.
"The NiCo2Se4 nanotube electrode presented a much better electrochemical performance in terms of cyclic stability and rate capability than other tested electrodes, including Ni3Se4 and Co3Se4. This is because the unique nanotube structure of NiCo2Se4 and the synergy offered by the co-presence of two metals," said Wang.
These monometallic counterparts, Ni3Se4 and Co3Se4 were not as successful as the bimetallic NiCo2Se4, simply because of the way the two metals (Ni and Co) interact together. NiCo2Se4 also had a higher capacity, which is very beneficial for maintaining cyclic stability and high-rate performance.
"This work offers new insights into the design of micro/nano-structured binary metal selenides as anodes for potassium-ion batteries with extraordinary potassium ion storage performance," said Wang.

 Previous page
Previous page Back to top
Back to top







