In a new Nature
Communications study, Columbia Engineering researchers report that they
have built highly conductive, tunable single-molecule devices in which the
molecule is attached to leads by using direct metal-metal contacts. Their novel
approach uses light to control the electronic properties of the devices and
opens the door to broader use of metal-metal contacts that could facilitate
electron transport across the single-molecule device.
As devices continue to shrink, their electronic components must also be miniaturized. Single-molecule devices, which use organic molecules as their conductive channels, have the potential to resolve the miniaturization and functionalization challenges faced by traditional semiconductors. Such devices offer the exciting possibility of being controlled externally by using light, but—until now—researchers have not been able to demonstrate this.
"With this work, we've unlocked a new dimension in molecular electronics, where light can be used to control how a molecule binds within the gap between two metal electrodes," said Latha Venkataraman, a pioneer in molecular electronics and Lawrence Gussman Professor of Applied Physics and professor of chemistry at Columbia Engineering. "It's like flipping a switch at the nanoscale, opening up all kinds of possibilities for designing smarter and more efficient electronic components."
Venkataraman's group has been studying the fundamental properties of single-molecule devices for almost two decades, exploring the interplay of physics, chemistry, and engineering at the nanometer scale. Her underlying focus is on building single-molecule circuits, a molecule attached to two electrodes with varied functionality, where the circuit structure is defined with atomic precision.
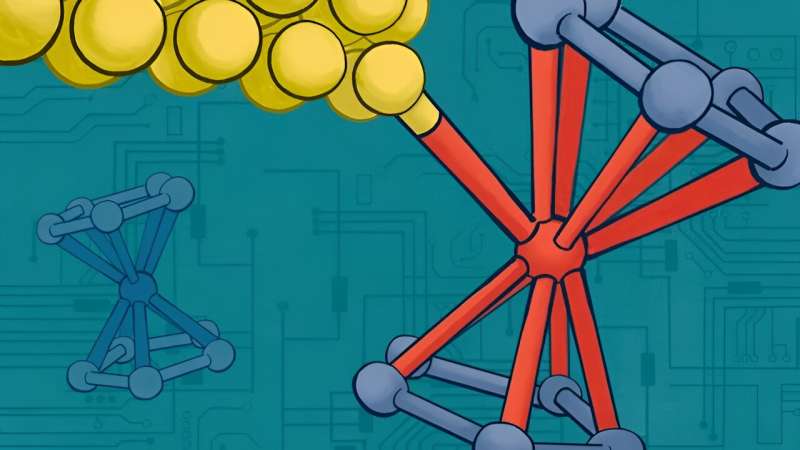
Her group, as well as those creating functional devices with graphene, a carbon-based two-dimensional material, have known that making good electrical contacts between metal electrodes and carbon systems is a major challenge. One solution would be to use organo-metallic molecules and devise methods to interface electrical leads to the metal atoms within the molecule. Towards this goal, they decided to explore the use of organo-metallic iron-containing ferrocene molecules, which are also considered to be tiny building blocks in the world of nanotechnology.
Just like LEGO pieces can be stacked together to create complex structures, ferrocene molecules can be used as building blocks to construct ultra-small electronic devices. The team used a molecule terminated by a ferrocene group comprising two carbon-based cyclopentadienyl rings that sandwich an iron atom.
They then used light to leverage the electrochemical properties of the ferrocene-based molecules to form a direct bond between the ferrocene iron center and the gold (Au) electrode when the molecule was in an oxidized state (i.e., when the iron atom had lost one electron). In this state, they discovered that ferrocene could bind to the gold electrodes used to connect the molecule to the external circuitry. Technically, oxidizing the ferrocene enabled the binding of an Au0 to a Fe3+ center.
"By harnessing the light-induced oxidation, we found a way to manipulate these tiny building blocks at room temperature, opening doors to a future where light can be used to control the behavior of electronic devices at the molecular level," said the study's lead author Woojung Lee, who is a Ph.D. student in Venkararaman's lab.
Venkataraman's new approach will enable her team to extend the types of molecular terminations (contact) chemistries they can use for creating single-molecule devices. This study also shows the ability to turn on and off this contact by using light to change the oxidation state of the ferrocene, demonstrating a light-switchable ferrocene-based single-molecule device. The light-controlled devices could pave the way for the development of sensors and switches that respond to specific light wavelengths, offering more versatile and efficient components for a wide range of technologies.

 Previous page
Previous page Back to top
Back to top







