Researchers
have developed a safer, cheaper, better performing and more flexible battery
option for wearable devices. A paper describing the "recipe" for
their new battery type was published in the journal Nano Research Energy on
June 3.
Fitness trackers. Smart watches. Virtual-reality headsets. Even smart clothing and implants. Wearable smart devices are everywhere these days. But for greater comfort, reliability and longevity, these devices will require greater levels of flexibility and miniaturization of their energy storage mechanisms, which are often frustratingly bulky, heavy and fragile. On top of this, any improvements cannot come at the expense of safety.
As a result, in recent years, a great deal of battery research has focused on the development of "micro" flexible energy storage devices, or MFESDs. A range of different structures and electrochemical foundations have been explored, and among them, aqueous micro batteries offer many distinct advantages.
Aqueous batteries—those that use a water-based solution as an electrolyte (the medium that allows transport of ions in the battery and thus creating an electric circuit) are nothing new. They have been around since the late 19th century.
However, their energy density—or the amount of energy contained in the battery per unit of volume—is too low for use in things like electric vehicles as they would take up too much space. Lithium-ion batteries are far more appropriate for such uses.
At the same time, aqueous batteries are much less flammable, and thus safer, than lithium-ion batteries. They are also much cheaper. As a result of this more robust safety and low cost, aqueous options have increasingly been explored as one of the better options for MFESDs. These are termed aqueous micro batteries, or just AMBs.
"Up till now, sadly, AMBs have not lived up to their potential," said Ke Niu, a materials scientist with the Guangxi Key Laboratory of Optical and Electronic Materials and Devices at the Guilin University of Technology—one of the lead researchers on the team. "To be able to be used in a wearable device, they need to withstand a certain degree of real-world bending and twisting. But most of those explored so far fail in the face of such stress."
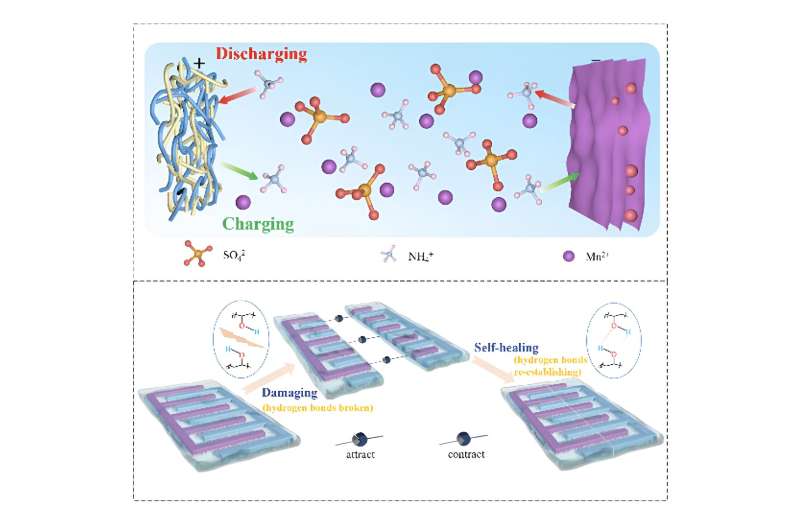
To overcome this, any fractures or failure points in an AMB would need to be self-healing following such stress. Unfortunately, the self-healing AMBs that have been developed so far have tended to depend on metallic compounds as the carriers of charge in the battery's electric circuit.
This has the undesirable side-effect of strong reaction between the metal's ions and the materials that the electrodes (the battery's positive and negative electrical conductors) are made out of. This in turn reduces the battery's reaction rate (the speed at which the electrochemical reactions at the heart of any battery take place), drastically limiting performance.
"So we started investigating the possibility of non-metallic charge carriers, as these would not suffer from the same difficulties from interaction with the electrodes," added Junjie Shi, another leading member of the team and a researcher with the School of Physics and Center for Nanoscale Characterization & Devices (CNCD) at the Huazhong University of Science and Technology in Wuhan.
The research team alighted upon ammonium ions, derived from abundantly available ammonium salts, as the optimal charge carriers. They are far less corrosive than other options and have a wide electrochemical stability window.
"But ammonium ions are not the only ingredient in the recipe needed to make our batteries self-healing," said Long Zhang, the third leading member of the research team, also at CNCD.
For that, the team incorporated the ammonium salts into a hydrogel—a polymer material that can absorb and retain a large amount of water without disturbing its structure. This gives hydrogels impressive flexibility—delivering precisely the sort of self-healing character needed. Gelatin is probably the most well-known hydrogel, although the researchers in this case opted for a polyvinyl alcohol hydrogel (PVA) for its great strength and low cost.
To optimize compatibility with the ammonium electrolyte, titanium carbide—a "2D" nanomaterial with only a single layer of atoms—was chosen for the anode (the negative electrode) material for its excellent conductivity. Meanwhile, manganese dioxide, already commonly used in dry cell batteries, was woven into a carbon nanotube matrix (again to improve conductivity) for the cathode (the positive electrode).
Testing of the prototype self-healing battery showed it exhibited excellent energy density, power density, cycle life, flexibility, and self-healing even after ten self-healing cycles.
The team now aims to further develop and optimize their prototype in preparation for commercial production.

 Previous page
Previous page Back to top
Back to top







