Viral vectors hold much potential
for gene editing and gene therapy, but there is a pressing need to develop
quality control methods to minimize potential side effects on patients.
Addressing this, researchers from Japan developed a nanosensing-based approach
that can differentiate between functional and faulty viral vectors at the
single-particle level. This convenient and inexpensive technique will hopefully
get us one step closer to advancing treatments for genetic disorders.
Over the past few decades, there has been remarkable progress in genetic manipulation technologies, bringing us closer to the point where genes can be modified in vivo. Such tools would open up the way to gene therapy, ushering in a new era in medicine. Thus far, the most promising strategies for gene therapy involve leveraging the existing molecular machinery found in viruses.
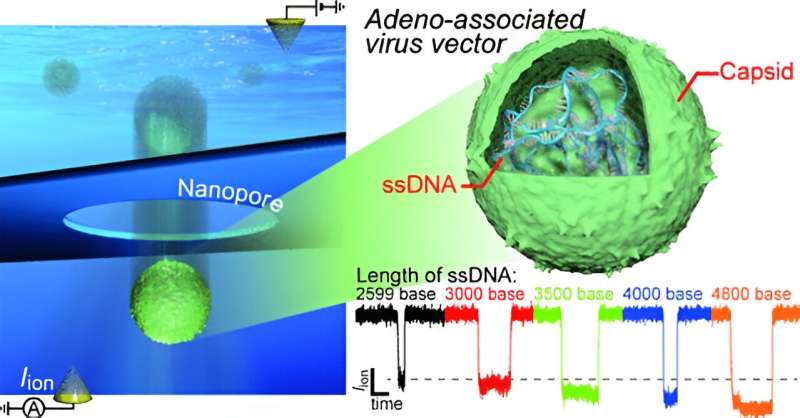
In particular, adeno-associated virus (AAV) vectors have recently garnered significant attention from the scientific community, given their potential to serve as nucleic acid vaccines for diseases such as COVID-19. However, during AAV vector manufacturing, some particles may carry only a partial copy of the intended genome, while others may be empty. These defective vectors can lead to unexpected side effects, underscoring the urgent need for robust quality control methods in their production.
Addressing this challenge, a team of researchers from Japan recently reported a novel nanosensing technique to measure viral vector characteristics. Their findings were presented in their latest paper, published online on 5 June, 2024, in ACS Nano.
The team includes Associate Professor Makusu Tsutsui and Professor Tomoji Kawai from the Institute of Scientific and Industrial Research at Osaka University; Lecturer Akihide Arima from the Institute of Nano-Life-Systems at Nagoya University; Specially Appointed Professor Yoshinobu Baba from the Institute of Nano-Life-Systems Institutes of Innovation for Future Society, Nagoya University; Project Researcher Mikako Wada, Assistant Professor Yuji Tsunekawa and Professor Takashi Okada, all from the Institute of Medical Science at The University of Tokyo.
The proposed approach involves measuring the ionic current that flows through a nanopore opening when a voltage differential is applied to a solution containing AAVs. When the nanopore is unobstructed, the measured ionic current is relatively constant. But when a viral particle passes through the nanopore, the flow of ions is partially blocked for a brief moment, producing a spike or pulse in the ionic current readout.
Interestingly, because AAV vectors with full genomes are heavier and slightly bulkier than empty or partially filled vectors, it's possible to discriminate between them as they pass through the nanopore—faulty vectors produce a "signature" in the measured ionic current that is noticeably different from that of full-genome vectors.
The team verified this through experiments, finite-element simulations, and theoretical analyses. "By designing a sensor with an optimal structure, we identified for the first time the gene-derived sub-nanometer-scale differences in the size of the viral vectors," explains Tsutsui.
This technique allows for the convenient and inexpensive quality control of AAV vectors, which thus far has relied on complex methods such as mass photometry, transmission electron microscopy, and analytical ultracentrifugation.
"The present work may revolutionize medicine by providing a tool for preparing AAV vectors with ultra-high quality for safe and effective gene therapy," highlights Tsunekawa. "It may be key in the development of production and purification systems for AAV vectors," he adds.
Moreover, beyond AAV vectors, this approach holds promise for studying other types of viral vectors, potentially opening new avenues for effective gene therapies and advancing our understanding of viral biology. Moreover, ensuring the high quality of clinically used AAV vectors could allow for lower patient doses, thereby minimizing side effects.

 Previous page
Previous page Back to top
Back to top







