No
molecule stands alone—they need others, at least when it comes to being able to
display useful photophysical, electronic, and chemical properties. When
individual molecules combine into an aggregate, or a complex of two or more
molecules, they become much more than the sum of their individual parts.
Photoactive molecular aggregates, though—complexes of two or more chromophores, which are molecules that absorb light at certain wavelengths, thereby displaying color—go where isolated molecules do not.
Due to the favorable interactions between molecules, these aggregates are of interest for biomedical, solar-energy-harvesting, and light-generating technologies.
That is because—in natural photosynthesis and in bioinspired technological applications—photoactive aggregates are efficient at energy transfer, the transport of solar energy from one place to another. For instance, in natural photosynthesis, the most widespread energy conversion system on our planet, aggregates efficiently transfer the energy from where the light is absorbed to where it is converted into charges for electricity or chemicals for fuel production.
National Renewable Energy Laboratory (NREL) researchers have synthesized two new compounds and studied how the properties of the individual molecules contribute to the—often unexpected—properties of the larger aggregates.
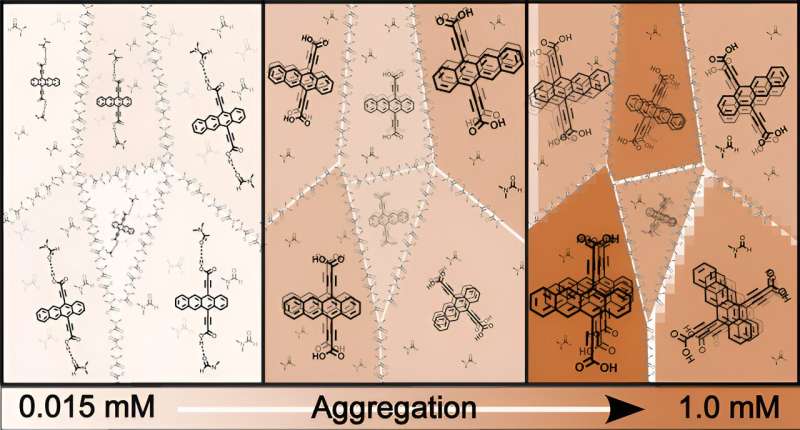
The team synthesized tetracene diacid (Tc-DA) and a dimethyl ester analog (Tc-DE) designed to prevent intermolecular hydrogen bonding while preserving Tc-DA's core electronics.
The results are described in the Journal of the American Chemical Society in a paper titled "Tetracene Diacid Aggregates for Directing Energy Flow toward Triplet Pairs."
"The goal of this fundamental study was to decipher which molecular properties dictate the eventual emergent properties of the collective ensemble where the whole is greater than the sum of the individual parts, similar to putting seemingly unrelated puzzle pieces together and an unexpected image emerges," said NREL's Justin Johnson, senior scientist.
"For molecular-based light harvesting architectures that aim to use unconventional mechanisms to more efficiently use the solar spectrum than typical solar cells, it's the collective properties that determine efficiency."
"Tc-DA was created to exploit intermolecular hydrogen-bonding interactions at semiconductor surfaces to well-ordered monolayers," said NREL's Nicholas Pompetti, postdoctoral researcher.
"However, we found that we could control the aggregation of Tc-DA as it approached the surface through solvent and concentration choices. This opened up insights about tetracene-based aggregates and how their size and structure provide promising pathways for their use in light-harvesting applications."
In a given solvent environment, strong intermolecular interactions direct stable and deterministic aggregation. However, strong but uncontrolled interactions might lead to the formation of large aggregates that may weaken solubility.
On the other hand, weak interactions spur dissociation with molecules acting as monomers. Fortunately for Tc-DA, the degree of aggregation can be finely controlled, ranging from monomers to stable larger order aggregates by changing concentration or solvent system.
Tetracene and its derivatives are prime candidates for singlet fission (SF), a process that may improve photoconversion efficiency by reducing wasteful heat production and relies on specific molecular dispositions that aggregates can achieve. Researchers used 1H nuclear magnetic resonance (NMR) spectroscopy, computational modeling, and concentration-dependent optical behavior to investigate the likely aggregate structure of Tc-DA and Tc-DE.
Steady-state spectroscopy analysis allowed them to observe absorption behavior and emission profiles of the aggregates. Computational modeling using density functional theory (performed by Kori Smyser and Sandeep Sharma at the University of Colorado Boulder), in combination with the NMR results, informed the researchers of the likely orientation of molecules within an aggregate structure.
Researchers then examined the impacts of aggregation on Tc-DA's excited-state dynamics using transient absorption spectroscopy.
"The excited-state dynamics were surprisingly sensitive to crossing a well-defined threshold of concentration, almost like going through a phase transition for a pure material," Johnson said.
As the size and structure of the aggregates are important to light harvesting, researchers systematically varied solvent polarity and concentration in solution to analyze the well-defined tetracene aggregates and their behaviors, including the potentially important singlet fission.
The researchers found that noncovalent tetracene-based aggregates beyond a dimer were stabilized at certain solvent polarities and concentrations, rapidly forming charge transfer and multiexcitonic states, which are desirable species for delivering charges (sometimes multiple units) to an electrode or catalyst.
The combination of NMR, computational studies, and spectroscopic results allowed the researchers to describe aggregate structures not often seen in solution-phase polyacenes.
"Controlling the landscape through molecular design and the associated solvent clearly allows us to dictate what the electrons do when they are photoexcited," Johnson said.
"Nature uses hydrogen bonds in many types of aggregated architectures to tune energy landscapes in a similar fashion, like funneling water to a reservoir. Bringing such principles to artificial light-harvesting systems with the potential for controlling multiexcitons is a logical pursuit that is leading to interesting consequences."

 Previous page
Previous page Back to top
Back to top







