The
goal of sustainable chemistry has motivated chemists to use renewable energy in
chemical reactions, minimizing hazardous waste, and maximizing atom economy.
Nature provides a blueprint with photosynthesis, in which carbohydrates are
produced from carbon dioxide and water under sunlight irradiation.
However, by relying on a complex system involving multiple enzymes and light-harvesting antennas, this process has intrinsically low solar energy conversion efficiency. Artificial photosynthetic systems have been a long-standing scientific pursuit and offer potential solutions to sustainable chemistry.
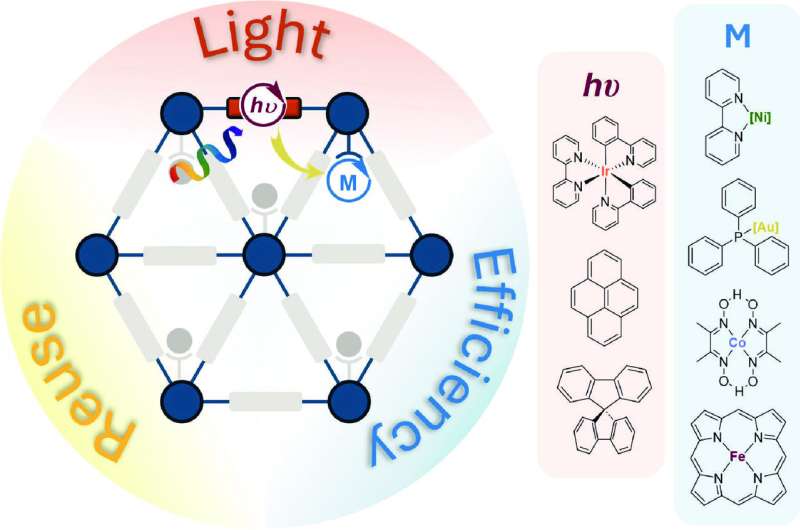
A team at the University of Chicago, led by Prof. Wenbin Lin, has been working on developing artificial photocatalytic systems using framework materials—a class of porous materials formed by periodic bonding of metal and organic building blocks.
By using cutting-edge techniques to characterize these materials, the researchers have gained a deep understanding of how such artificial systems function at the molecular level. This knowledge has allowed them to fine-tune the materials for various light-driven reactions.
In a minireview published in Carbon Future on September 13, 2024, the researchers summarized their recent accomplishments in artificial photosynthesis and photocatalysis to highlight key advances and future opportunities.
"Nature performs precision chemistry in organisms to make complex molecules, often by sacrificing efficiency," said Prof. Wenbin Lin.
"We need to surpass nature to address the challenges we face today, and fortunately, with precise control over the structures and compositions of framework materials, we have developed artificial systems that significantly outperform their homogeneous analogs."
The review illustrates how chemical modifications of framework materials can fine-tune their performances in photosynthesis-like reactions.
To accomplish these goals, the team identified essential components and verified their roles. Photosensitizers, like chlorophylls, absorb light energy. Catalysts, like enzymes, use this energy to drive chemical reactions. These photosensitizers and catalysts with carefully matched energy and electron transfer kinetics were incorporated into framework materials.
"Incorporating the right photosensitizers and catalysts into framework materials can enhance their performances by more than an order of magnitude over simple mixtures of photosensitizers and catalysts in solutions," Lin explained.
The team demonstrated significant improvements in a dozen types of photocatalytic reactions using these materials. The enhancement stems from a "pre-organization" effect, also found in natural systems, where photosensitizers and catalysts are arranged at specific positions to boost chemical reactions.
The framework materials are easily recovered from the reaction mixtures by centrifugation or filtration. The recovered materials are used in subsequent reactions without loss of catalytic activities. In one example, the framework material was used in eight cycles of one-pot synthesis of a cardiotonic agent without degradation of catalytic performance.
"We believe this breakthrough holds great potential for sustainable synthesis of pharmaceuticals and other value-added products, and these research efforts will contribute to a more sustainable future," Lin said.
"The principles we've learned here can be applied to many other systems." The team hopes their review will inspire other researchers to rationally design other catalytic materials at the molecular level.
The first author was Yingjie Fan (Ph.D. '24, now a postdoctoral scholar at UC Berkeley).

 Previous page
Previous page Back to top
Back to top







