Under the leadership of Prof. Dr.
Francesco Ciucci from the University of Bayreuth, a German–Chinese research
team has developed a new method for the electrochemical splitting of water.
This not only accelerates the production of hydrogen for technology and
industry but also makes it more sustainable. The researchers published their
findings in Nature Nanotechnology.
Hydrogen is of crucial importance to technology and industry due to its unique properties: It is the lightest chemical element, has an extremely high energy density, and is an emission-free fuel, as water is the only byproduct of its combustion. This makes hydrogen a highly attractive clean energy source. However, its production is still extremely energy intensive.
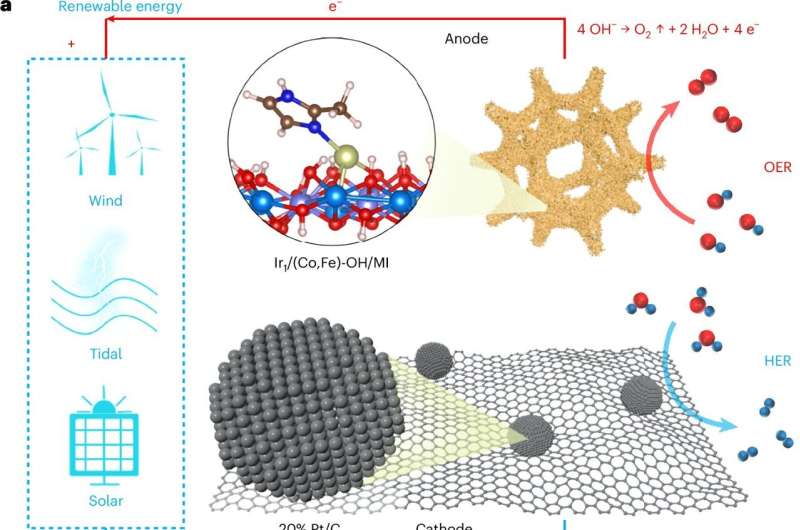
Hydrogen can be produced via electrochemical water splitting, where electrodes in water are subjected to an electric current. Energy-efficient and sustainable hydrogen production through electrochemical water splitting with renewable electricity could significantly improve the sustainability of this energy source.
One of the biggest challenges in electrochemical water splitting is the so-called oxygen evolution reaction (OER), a sluggish reaction in which water molecules are broken down into their individual components—oxygen and hydrogen. The OER can be accelerated by using noble metal catalysts; however, these metals are expensive and scarce, and speeding up the reaction requires additional energy (known as overpotential).
This challenge has been addressed by a research team, consisting of members from various Chinese research institutions and led by Prof. Dr. Ciucci, Chair of Electrode Design for Electrochemical Energy Systems at the University of Bayreuth. They developed an innovative method for electrochemical water splitting.
This approach employs atomically dispersed iridium as reaction accelerators, coupling them with dimethylimidazole and cobalt-iron hydroxide. The key innovation lies in the geometric arrangement of these components, which are configured in an out-of-plane orientation, optimizing performance and efficiency.
This innovative approach significantly increases OER activity and also exhibits an ultra-low overpotential. Additionally, it reduces the use of noble metals, as only individual iridium atoms are used, and it positively impacts the stability of the acceleration reaction.
"Our study represents a significant step forward in developing efficient, cost-effective OER acceleration for sustainable hydrogen production. By overcoming the key challenge of current technology, our results have the potential to drive the global transition towards clean energy solutions," says Ciucci, the senior author of the study.

 Previous page
Previous page Back to top
Back to top







