Who could forget the scene in Terminator 2: Judgement Day where the shape-shifting T-1000 reassembles itself from thousands of blobs of molten metal? Researchers from North Carolina State University (NCSU) have taken the first steps to such science fiction becoming reality by developing a way to control the surface tension of liquid metals with the application of very low voltages. This may offer opportunities in a new field of morphing electronic circuits, self-healing electronics, or – one day – maybe even self-assembling terminator-style robots.
The liquid metal used by the researchers was an alloy of gallium and indium. Gallium is liquid just above room temperature at about 29° C (84° F), while Indium has a much higher melting point at around 156° C (312° F), yet when mixed together, they form an alloy that is liquid at room temperature. In other words, a eutectic alloy – one that is composed of metals with disparate melting points that, when combined, melt as a whole at a specific temperature.
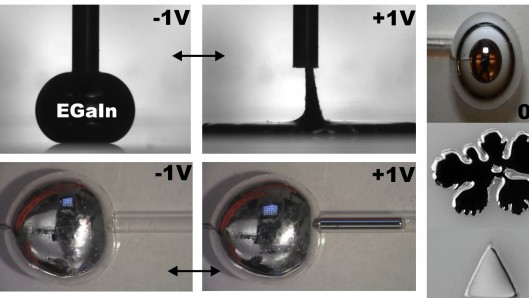
Another important aspect of this eutectic alloy, and one that the researchers sought to exploit in their experiments, is its exceptionally high surface tension of approximately 500 millinewtons per meter (mN/m). The consequence of this is that a blob of this alloy resting on a surface will tend to form an almost spherical ball and hold its shape if undisturbed.
Researchers found that if they applied a small voltage (less than one volt) in water to such a blob of the alloy, they were able reduce the surface tension significantly, resulting in the molten metal spreading and flattening out. When the voltage was removed, the high surface tension returned, and the blob once more took on its spherical shape.
The amount of surface tension could also be varied dependent upon that voltage, making the blob more or less viscous depending on the electrical charge applied to it. In other words, the liquid metal could be held in various states of liquidity from its original 500 mN/m right down to 2 mN/m, and anything in between.
The significance to this demonstration is that the metal could be made to flow in and out of variously shaped capillaries or molds, allowing the alloy to take on different contours. If these shapes were that of an antenna, for example, then the metal could behave as a highly-variable or tunable antenna capable of morphing its shape to receive or transmit a wide range of different wavelengths all from the same component.
NCSU researchers have experimented with other versions of shape-shifting antennas in the past, but this is the first time that they have utilized electricity – rather than mechanical deformation – to alter the shape.
"The resulting changes in surface tension are among the largest ever reported, which is remarkable considering it can be manipulated by less than one volt," said Dr. Michael Dickey, an associate professor of chemical and biomolecular engineering at NCSU and lead author of the research. "We can use this technique to control the movement of liquid metals, allowing us to change the shape of antennas and complete or break circuits. It could also be used in microfluidic channels, MEMS, or photonic and optical devices. Many materials form surface oxides, so the work could extend beyond the liquid metals studied here."
This technique – a version of electrohydrodynamics in which electric currents affect the movement of liquid metals – relies on an oxide "skin" forming on the surface of the alloy when a voltage is applied that behaves as a surfactant, lowering the surface tension between the metal and the surrounding fluid.
This current research builds on experiments previously carried out by the NCSU lab when they revealed a process for "3-D printing" liquid metals where, unlike this experiment in water, they created the oxide layer in open air to assist the liquid metal in retaining its shape.

 Previous page
Previous page Back to top
Back to top







