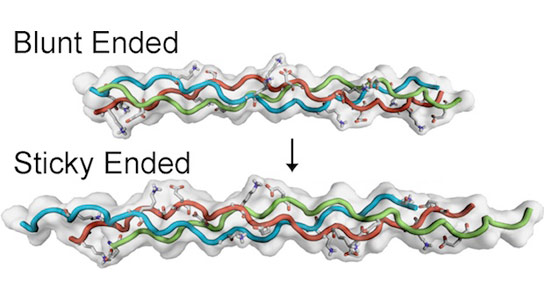
The two papers in the Journal of the American Chemical Society, the first published in May and the second this month, show precisely how mimetic peptides developed at Rice may be aligned to form helices with sticky ends that allow them to aggregate into fibers or gels.
“We proposed in a Nature Chemistry paper (in 2011) that peptides self-assemble in an offset fashion that creates sticky ends,” Hartgerink said. “And those sticky ends can then propagate. But even in the review of that paper, the mechanism took a fair amount of flak because there had been no reports in the literature that a collagen system could, in fact, create this offset.”
It has taken several years and these two papers to prove the critics wrong, he said.
Collagen forms from helical arrangements of three protein chains, also called peptides. The chains, like all proteins, consist of amino acid like glycine and proline (two primary components of collagen) arranged in a certain order. Scientists like Hartgerink design custom nanoscale chains by carefully arranging the amino acids and their positive and negative charges.
In the right order, the charged amino acids crosslink into what Hartgerink calls axial salt bridges, non-covalent bonds that hold the helices together with the help of stabilizing hydrogen bonds. “Most of the work we’ve done on collagen has been to define those axial charged pairs and then utilize them to do different things,” he said.
The ends of these helices can be blunt or sticky, Hartgerink said, depending on how the chains are staggered, or offset. An offset of one amino acid is blunt and not sticky at all. The greater the offset, the stickier it becomes.
The first of the new papers by Hartgerink, graduate students Abihishek Jalan and Katherine Jochim demonstrated the self-assembly of standalone, sticky-ended triple helices with offsets of four amino acids. They allowed the researchers to obtain details of the structure through nuclear magnetic resonance (NMR).
“We chose to use a really short offset because as soon as we make it a little bit bigger, it becomes stickier and fibers start forming,” Hartgerink said. “As soon as you have a fiber, NMR doesn’t work any more. But NMR is the go-to analytical tool, and in this case we were able to show at a molecular level that the offset takes place.”
For the second paper with graduate student Biplab Sarkar and former graduate student Lesley O’Leary, “We did the reverse of that and synthesized a series of peptides with large sticky ends that drive fiber assembly,” Hartgerink said.
The researchers made two classes of synthetic collagen peptides with the same chemical components but different arrangements and showed those with extensive sticky ends quickly self-assembled into fibers. Those with misaligned sequences either formed amorphous aggregates or remained separated in solution.
“We chose to satisfy the critics by breaking our work into two studies. One was to demonstrate at the molecular level that these charged pairs are powerful and can drive this kind of offset, which hadn’t been demonstrated before, and that the resulting helices are potentially sticky,” he said. “Then, in the second study, we made them actually sticky and showed that when you use charged pairs properly, you get fibers. And when you don’t, you don’t get fibers.”
Understanding the fine details of collagen assembly presents the possibility of synthetic collagens for specific functions, Hartgerink said. “A number of biomaterials use natural collagen, and there are advantages to replacing them with synthetic collagens,” he said. “One of the main advantages is that we move away from health and regulatory problems associated with using animal sources.”
He said synthetic collagen may also help answer important questions about its natural counterpart. “Collagen is an incredibly important central player in tissue behavior, but even some of the very basic ideas about collagen structure have eluded scientists for decades,” he said. “One of the things we’re pursuing now is generating libraries of collagen mimetic peptides to test them against known collagen-binding proteins and proteases, proteins that cut up collagen.
“I think the difficulty in understanding the mechanism is illustrated by the fact that it took two whole papers just to outline it,” Hartgerink said. “But these mechanistic studies will allow us to push the science forward and do more practical things.”
The National Science Foundation and the Robert A. Welch Foundation supported the research.

 Previous page
Previous page Back to top
Back to top







