We already knew that graphene was a highly useful material, but just how useful is it? Well, it turns out that even defective graphene may be valuable. According to a team of mostly-American scientists, improperly-formed graphene could find use in next-generation fuel cells. Among other things, those cells might allow electric cars to be recharged in the amount of time that it currently takes to refuel a gas-burning vehicle.
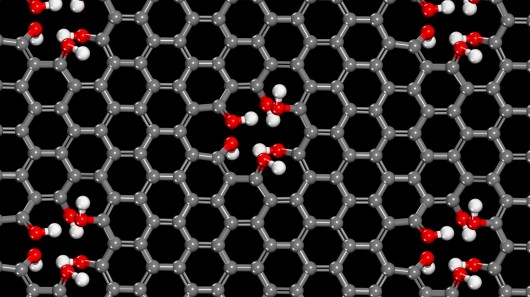
Graphene, as many people will already know, is a one-atom-thick layer of carbon atoms, linked together in a honeycomb pattern. Sometimes, however, its uniform structure is marred by gaps that form between adjacent atoms. We'll get back to those gaps in a moment.
Proton exchange membrane fuel cells, meanwhile, work by separating protons from hydrogen. They do so using a polymer electrolyte membrane, which only allows protons to pass through. Given that those membranes are hundreds of nanometers thick, however, it takes several minutes for the tiny protons to make their way through.
Graphene is of course much thinner, but in its regular form is impermeable to protons at room temperature. As the scientists discovered, however, the gaps in imperfect graphene are just the right size to allow protons in water to pass through – and those protons do so in just a few seconds. A "handful" of the holes per square micron of graphene were found to be sufficient for the process to work effectively.
"Our results will not make a fuel cell tomorrow, but it provides a mechanism for engineers to design a proton separation membrane that is far less complicated than what people had thought before," said lead scientist Franz M. Geiger, of Northwestern University. "All you need is slightly imperfect single-layer graphene."
Also taking part in the study were scientists from the University of Minnesota, Pennsylvania State University, Oak Ridge National Laboratory, the University of Virginia, and the University of Puerto Rico. A paper on the research was published this week in the journal Nature Communications.

 Previous page
Previous page Back to top
Back to top







