One potential clean energy future requires an economical, efficient, and relatively simple way to generate copious amounts of hydrogen for use in fuel-cells and hydrogen-powered vehicles. Often achieved by using electricity to split water molecules into hydrogen and oxygen, the ideal method would be to mine hydrogen from water using electricity generated directly from sunlight without the addition of any external power source. Hematite – the mineral form of iron – used in conjunction with silicon has shown some promise in this area, but low conversion efficiencies have slowed research. Now scientists have discovered a way to make great improvements, giving hope to using two of the most abundant elements on earth to efficiently produce hydrogen.
One potential clean energy future requires an economical, efficient, and relatively simple way to generate copious amounts of hydrogen for use in fuel-cells and hydrogen-powered vehicles. Often achieved by using electricity to split water molecules into hydrogen and oxygen, the ideal method would be to mine hydrogen from water using electricity generated directly from sunlight without the addition of any external power source. Hematite – the mineral form of iron – used in conjunction with silicon has shown some promise in this area, but low conversion efficiencies have slowed research. Now scientists have discovered a way to make great improvements, giving hope to using two of the most abundant elements on earth to efficiently produce hydrogen.
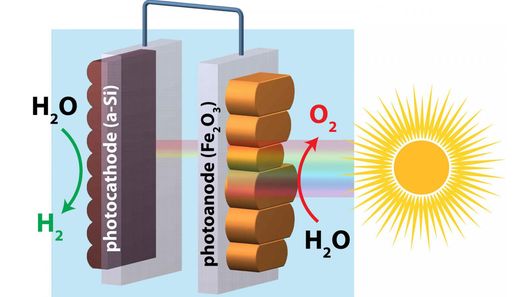
Hematite holds potential for use in low-power photoelectrochemical water splitting (where energy, in the form of light, is the input and chemical energy is the output) to release hydrogen due to its low turn-on voltage of less than 0.3 volts when exposed to sunlight. Unfortunately, that voltage is too low to initiate water-splitting so a number of improvements to the surface of hematite have been sought to improve current flow.
In this vein, researchers from Boston College, UC Berkeley, and China's University of Science and Technology have hit upon the technique of "re-growing" the hematite, so that a smoother surface is obtained along with a higher energy yield. In fact, this new version has doubled the electrical output, and moved one step closer to enabling practical, large-scale energy-harvesting and hydrogen generation.
"By simply smoothing the surface characteristics of hematite, this close cousin of rust can be improved to couple with silicon, which is derived from sand, to achieve complete water splitting for solar hydrogen generation," said Boston College associate professor of chemistry Dunwei Wang. "This unassisted water splitting, which is very rare, does not require expensive or scarce resources."
Working on previous work that realized gains in the photoelectrochemical turn-on voltage from the use of smooth surface coatings, the team re-assessed the hematite surface structure by employing a synchrotron particle accelerator at the Lawrence Berkeley National Laboratory. Concentrating on massaging the hematite's surface deficiencies to see if this would result in improvements, the researchers used physical vapor deposition to layer hematite onto a borosilicate glass substrate and create a photoanode. They then baked the devices to produce a thin, even film of iron oxide across their surfaces.
Subsequent tests of this new amalgam resulted in an immediate improvement in turn-on voltage, and a substantial increase in photovoltage from 0.24 volts to 0.80 volts. Whilst this new hydrogen harvesting process only realized an efficiency of 0.91 percent, it is the very first time that the combination of hematite and amorphous silicon has been shown to produce any meaningful efficiencies of conversion at all.
As a result, this research has shown that progress has been made towards the possibility of producing photoelectrochemical energy harvesting that is totally self-sufficient, uses abundantly available materials, and is easy to produce.
"This offers new hope that efficient and inexpensive solar fuel production by readily available natural resources is within reach," said Wang. "Getting there will contribute to a sustainable future powered by renewable energy."

 Previous page
Previous page Back to top
Back to top







