A new materials recipe has been developed for a battery-like hydrogen fuel cell that shields the nanocrystals from oxygen, moisture and contaminants while pushing its performance forward in key areas.
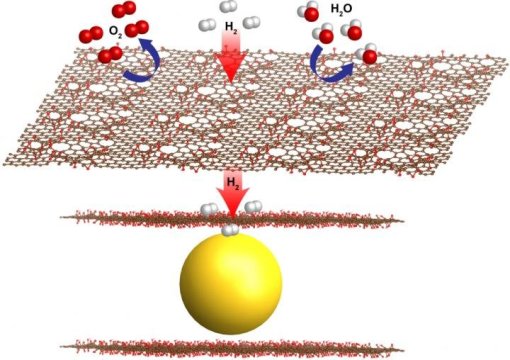
While there remain scientific challenges to making hydrogen-based energy sources more competitive with current automotive propulsion systems and other energy technologies, researchers at the U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) have developed a new materials recipe for a battery-like hydrogen fuel cell--which surrounds hydrogen-absorbing magnesium nanocrystals with atomically thin graphene sheets--to push its performance forward in key areas.
The graphene shields the nanocrystals from oxygen and moisture and contaminants, while tiny, natural holes allow the smaller hydrogen molecules to pass through. This filtering process overcomes common problems degrading the performance of metal hydrides for hydrogen storage.
These graphene-encapsulated magnesium crystals act as "sponges" for hydrogen, offering a very compact and safe way to take in and store hydrogen. The nanocrystals also permit faster fueling, and reduce the overall "tank" size.
"Among metal hydride-based materials for hydrogen storage for fuel-cell vehicle applications, our materials have good performance in terms of capacity, reversibility, kinetics and stability," said Eun Seon Cho, a postdoctoral researcher at Berkeley Lab and lead author of a study related to the new fuel cell formula, published recently in Nature Communications.
In a hydrogen fuel cell-powered vehicle using these materials, known as a "metal hydride" (hydrogen bound with a metal) fuel cell, hydrogen gas pumped into a vehicle would be chemically absorbed by the magnesium nanocrystaline powder and rendered safe at low pressures.
Jeff Urban, a Berkeley Lab staff scientist and co-author, said, "This work suggests the possibility of practical hydrogen storage and use in the future. I believe that these materials represent a generally applicable approach to stabilizing reactive materials while still harnessing their unique activity--concepts that could have wide-ranging applications for batteries, catalysis, and energetic materials."
The research, conducted at Berkeley Lab's Molecular Foundry and Advanced Light Source, is part of a National Lab Consortium, dubbed HyMARC (Hydrogen Materials--Advanced Research Consortium) that seeks safer and more cost-effective hydrogen storage, and Urban is Berkeley Lab's lead scientist for that effort.
The U.S. market share for all electric-drive vehicles in 2015, including full-electric, hybrids and plug-in hybrid vehicles, was 2.87 percent, which amounts to about 500,000 electric-drive vehicles out of total vehicle sales of about 17.4 million, according to statistics reported by the Electric Drive Transportation Association, a trade association promoting electric-drive vehicles.
Hydrogen-fuel-cell vehicles haven't yet made major in-roads in vehicle sales, though several major auto manufacturers including Toyota, Honda, and General Motors, have invested in developing hydrogen fuel-cell vehicles. Indeed, Toyota released a small-production model called the Mirai, which uses compressed-hydrogen tanks, last year in the U.S.
A potential advantage for hydrogen-fuel-cell vehicles, in addition to their reduced environmental impact over standard-fuel vehicles, is the high specific energy of hydrogen, which means that hydrogen fuel cells can potentially take up less weight than other battery systems and fuel sources while yielding more electrical energy.
A measure of the energy storage capacity per weight of hydrogen fuel cells, known as the "gravimetric energy density," is roughly three times that of gasoline. Urban noted that this important property, if effectively used, could extend the total vehicle range of hydrogen-based transportation, and extend the time between refueling for many other applications, too.
More R&D is needed to realize higher-capacity hydrogen storage for long-range vehicle applications that exceed the performance of existing electric-vehicle batteries, Cho said, and other applications may be better suited for hydrogen fuel cells in the short term, such as stationary power sources, forklifts and airport vehicles, portable power sources like laptop battery chargers, portable lighting, water and sewage pumps and emergency services equipment.
Cho said that a roadblock to metal hydride storage has been a relatively slow rate in taking in (absorption) and giving out (desorption) hydrogen during the cycling of the units. In fuel cells, separate chemical reactions involving hydrogen and oxygen produce a flow of electrons that are channeled as electric current, creating water as a byproduct.
The tiny size of the graphene-encapsulated nanocrystals created at Berkeley Lab, which measure only about 3-4 nanometers, or billionths of a meter across, is a key in the new fuel cell materials' fast capture and release of hydrogen, Cho said, as they have more surface area available for reactions than the same material would at larger sizes.
Another key is protecting the magnesium from exposure to air, which would render it unusable for the fuel cell, she added.
Working at The Molecular Foundry, researchers found a simple, scalable and cost-effective "one pan" technique to mix up the graphene sheets and magnesium oxide nanocrystals in the same batch. They later studied the coated nanocrystals' structure using X-rays at Berkeley Lab's Advanced Light Source. The X-ray studies showed how hydrogen gas pumped into the fuel cell mixture reacted with the magnesium nanocrystals to form a more stable molecule called magnesium hydride while locking out oxygen from reaching the magnesium.
"It is stable in air, which is important," Cho said.
Next steps in the research will focus on using different types of catalysts--which can improve the speed and efficiency of chemical reactions--to further improve the fuel cell's conversion of electrical current, and in studying whether different types of material can also improve the fuel cell's overall capacity, Cho said.

 Previous page
Previous page Back to top
Back to top







