The prospect of using sunlight to power our energy-intensive lifestyles has enough merit on its own, but what if we could suck carbon dioxide out of the atmosphere while we're at it? This two-pronged environmental panacea has inspired scientists eyeing a greener future, with artificial leaves, hybrid energy systems and moth-inspired photoelectrochemical cells just a few examples of how we are progressing toward this goal.
Scientists at Chicago's University of Illinois have been working with new kinds of chemicals with new kinds of properties to take these efforts to the next level. The key, they say, is to discover a new type of catalyst that can turn atmospheric CO2 into burnable fuels in an efficient and inexpensive way.
In pursuit of this, the team was working with a set of nanostructured compounds called transition metal dichalcogenides, or TMDCs. It happened upon one TMDC called nanoflake tungsten diselenide which, when paired with water and a particular ionic liquid as the electrolyte, worked 1,000 times faster than the expensive metals usually used in these CO2 reduction technologies. The fact that it is about 20 times cheaper didn't hurt either.
"The active sites of the catalyst get poisoned and oxidized," says Amin Salehi-Khojin, senior author on the study. "The combination of water and the ionic liquid makes a co-catalyst that preserves the catalyst's active sites under the harsh reduction reaction conditions."
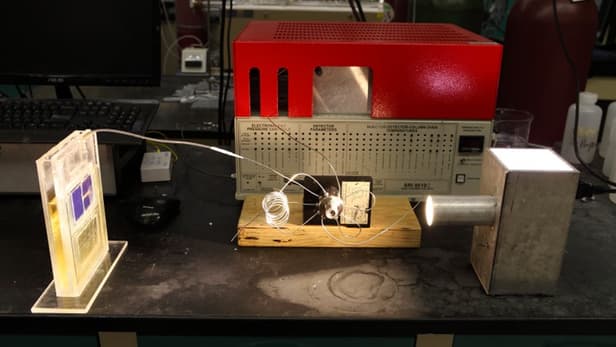
The solar cell itself is made up of two silicon triple-junction photovoltaic cells measuring 18 cm sq (2.8 in sq) to harvest the light, with the co-catalyst system on the cathode side and cobalt oxide in potassium phosphate electrolyte on the anode side.
When 100 W of light per meter squared hits the cell, it kicks off a chemical reaction where hydrogen and carbon monoxide gas are produced from the cathode. Free oxygen and hydrogen ions are generated at the anode. This reaction creates synthesis gas, or syngas, which can be burned as is or turned into diesel and other hydrocarbon fuels.
According to the team, the solar cell could be adapted to large-scale use such as solar farms, along with smaller applications. One day, it might even provide power on Mars if water can be found there, as the planet's atmosphere is largely carbon dioxide.
"The new solar cell is not photovoltaic — it's photosynthetic," says Salehi-Khojin. "Instead of producing energy in an unsustainable one-way route from fossil fuels to greenhouse gas, we can now reverse the process and recycle atmospheric carbon into fuel using sunlight."

 Previous page
Previous page Back to top
Back to top







