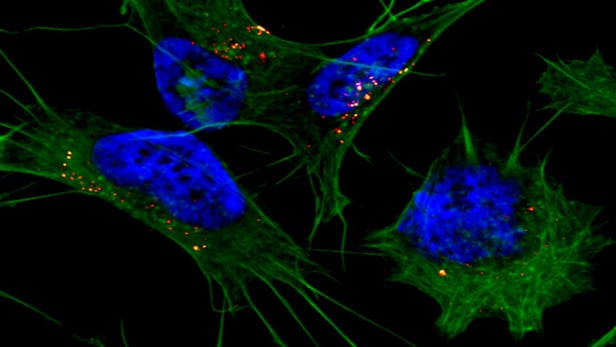
Scientists have established a rough outline of one way in which Alzheimer's disease takes hold, and bit by bit they are starting to fill in the detail. Key players in this process are what are known as amyloid beta proteins which form in clumps, evolve into plaques and cause irreversible damage to the brain. New research has revealed the crafty maneuver that these peptides use to slip past the cell's defenses whereby they first change shape to form stacks of long, flat sheets, a finding that may offer up new opportunities to intervene in the onset of the disease.
The accumulation of amyloid beta proteins is considered a precursor to Alzheimer's. Long before telltale signs like memory loss and compromised communication skills begin to appear, these peptides team up to attack the synapses of the neurons and destroy brain cell connections, all the while forming plaques that correspond with the more dramatic decline in brain function later on.
It is known that when amyloid beta proteins aggregate and whittle away at the neurons they do so in the shape of these sheet-like structures, sometimes called amyloid beta oligomers. These can appear more than a decade before the aforementioned plaques are detected, and promisingly, recent research has resulted in diagnostics like MRI techniques and blood tests that could be used to spot them much earlier on. What the new study has revealed is the shapeshifting proteins actually present themselves like this as a way of gaining entry to the cell.
"What we found is that this structural change is required for the Abeta (amyloid beta) to enter neuronal cells and that single non-aggregated Abeta molecules first aggregate and change into beta-sheet structure before they are taken up – not after," Jan Bieschke, a biomedical engineer at Washington University in St. Louis and co-author of the new study tells New Atlas.
Bieschke and his team of collaborating researchers from Germany made the discovery by first building different types of amyloid beta structures in the lab and using fluorescence microscopy to track their uptake by the cell. In a second, separate experiment, they consolidated their findings by feeding non-aggregated amyloid beta proteins to the cells and used fluorescence resonance energy transfer to see how they gather to pick the lock.
The process, which the researchers liken to the construction of a layered cake, is what allows the harmful amyloid beta clusters to be absorbed into the cell. How exactly they get their toxicity is still up for debate, though there is evidence of amyloid beta proteins damaging the cell's mitochondria, the energy production center, and leading to cell death.
"Whether this is the primary mechanism of toxicity is still an open question, since there are other possible mechanisms for example when the amyloid beta interacts with the cell membrane," explains Bieschke. "All of these mechanisms are linked to the aggregation or 'cake forming' of the Abeta peptide."
With a new understanding of how the amyloid beta proteins gain entry, the researchers can now look into how it behaves once inside the cell and if its trickery has any bearing on how it interacts with the mitochondria.
"We will determine if we can see and measure the interaction with the mitochondria membrane, and if these structures are interacting with mitochondria the same way as with the outer cell membrane," says Bieschke. "Another question we will ask is: Can we manipulate the uptake or formation of these structures so they cannot enter the cell? This may be a therapeutic strategy to help future patients with Alzheimer's."

 Previous page
Previous page Back to top
Back to top







