Plastics have taken the world by
storm over the last century, finding applications in virtually every aspect of
our lives. However, the rise of synthetic polymers, which form the basis of
plastics, has contributed to many serious environmental issues. The worst of
these is the excessive use of petrochemical compounds and the disposal of
non-biodegradable materials without recycling; only 14% of all plastic waste is
recycled, which hardly puts a dent in the problem.
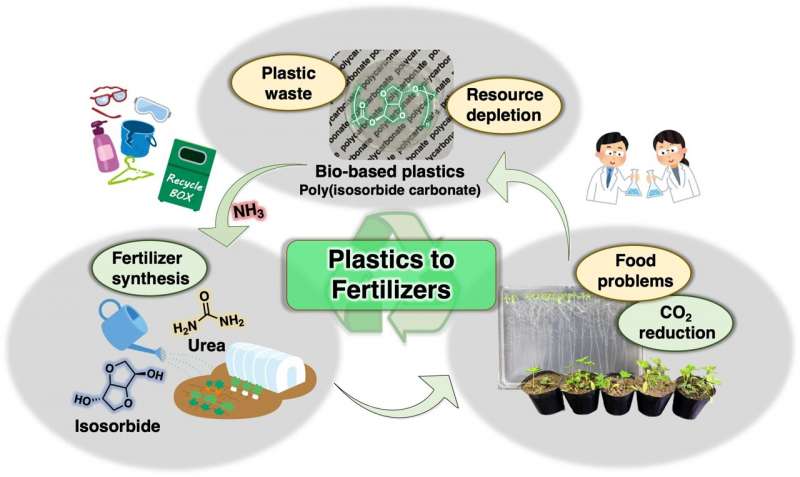
Solving the plastic conundrum requires "circular" systems, in which the source materials used to produce the plastics come full circle after disposal and recycling. At Tokyo Institute of Technology, a team of scientists led by Assistant Professor Daisuke Aoki and Professor Hideyuki Otsuka is pioneering a novel concept. In their new, environmentally friendly process, plastics produced using biomass (bioplastics) are chemically recycled back into fertilizers. This study will be published in Green Chemistry, a journal of the Royal Society of Chemistry focusing on innovative research on sustainable and eco-friendly technologies.
The team focused on poly (isosorbide carbonate), or PIC, a type of bio-based polycarbonate that has garnered much attention as an alternative to petroleum-based polycarbonates. PIC is produced using a non-toxic material derived from glucose called isosorbide (ISB) as a monomer. The interesting part is that the carbonate links that join the ISB units can be severed using ammonia (NH3) in a process known as ammonolysis. The process produces urea, a nitrogen-rich molecule that is widely used as a fertilizer. While this chemical reaction was no secret to science, few studies on polymer degradation have focused on the potential uses of all the degradation products instead of only the monomers.
First, the scientists investigated how well the complete ammonolysis of PIC could be conducted in water at mild conditions (30 degrees Celsius and atmospheric pressure). The rationale behind this decision was to avoid the use of organic solvents and excessive amounts of energy. The team carefully analyzed all the reaction products through various means, including nuclear magnetic resonance spectroscopy, the Fourier transform infrared spectroscopy, and gel permeation chromatography.
Although they managed to produce urea in this way, the degradation of PIC was not complete even after 24 hours, with many ISB derivatives still present. Therefore, the researchers tried increasing the temperature and found that complete degradation could be achieved in about six hours at 90 degrees Celsius. Dr. Aoki highlights the benefits of this approach: "The reaction occurs without any catalyst, demonstrating that the ammonolysis of PIC can be easily performed using aqueous ammonia and heating. Thus, this procedure is operationally simple and environmentally friendly from the viewpoint of chemical recycling."
Finally, as a proof-of-concept that all PIC degradation products can be directly used as a fertilizer, the team conducted plant growth experiments with Arabidopsis thaliana, a model organism. They found that plants treated with all PIC degradation products grew better than plants treated with just urea.
The overall results of this study showcase the feasibility of developing fertilizer-from-plastics systems. The systems can not only help fight off pollution and resource depletion but also contribute to meeting the world's increasing food demands. Dr. Aoki concludes on a high note, "We are convinced that our work represents a milestone toward developing sustainable and recyclable polymer materials in the near future. The era of 'bread from plastics' is just around the corner."

 Previous page
Previous page Back to top
Back to top







