Home-based continuous
glucose monitoring for diabetics up to now has had to trade ease of use, low
cost, and portability for a somewhat lower sensitivity—and thus
accuracy—compared to similar systems in clinics or hospitals. A team of
researchers has now developed a biosensor for such monitors that involves
"zero-dimensional" quantum dots (QDs) and gold nanospheres (AuNSs),
and no longer has to compromise on accuracy.
A paper describing the biosensor design and its enhanced performance appeared in the journal Nano Research on Nov. 9, 2022.
In recent years, the development of continuous glucose monitoring (CGM) technology has been a great boon for people with diabetes. Unlike pre-meal and pre-bedtime blood sugar testing, the real-time, rapid, and accurate detection of glucose levels of always-on CGM devices has significantly improved diabetic management.
Glucose trends are more easily tracked, making diet, exercise, and medicine changes to a diabetes care plan easier to implement throughout the day, and alarms go off when glucose levels climb too high or fall too low, sending information to the individual or to parents, partners, or caregivers.
CGMs typically work via a tiny biosensor embedded under the skin that measures glucose levels in the fluid between cells. This sensor checks such levels every few minutes and sends that information to a monitor. The monitor can also be connected to an insulin pump.
Various techniques for glucose detection have been developed, including colorimetry, infrared spectroscopy, fluorescence spectroscopy, and mass spectrometry. But for home-based operation rather than at a clinic or hospital, electrochemical glucose detection is the most widely accepted technique due to its rapid response, ease of use, low cost, and portability.
"It also has decent sensitivity, but not excellent sensitivity," said Huan Liu, a microelectronics specialist with the School of Optical and Electronic Information at Huazhong University of Science and Technology. "Not compared to other techniques used in a health care setting. So we wanted to see if we could bring a bit of a boost to that sensitivity and thus improve its accuracy."
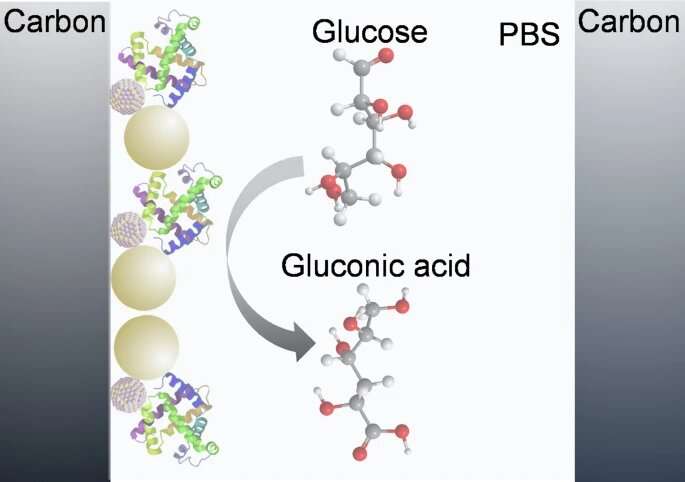
Electrochemical glucose sensors can be classified as enzyme-based sensors and non-enzyme-based sensors. For the enzyme-based glucose electrochemical sensors, glucose oxidase (GOx)—an enzyme that speeds up (catalyzes) oxidation-reduction chemical reactions—is widely used to oxidize glucose on the surface of the CGM sensor electrode.
The electrode attracts electrons from the glucose (oxidizing them), and in the process generates an electric current that varies depending on glucose levels. GOx is widely used for this purpose due to its high selectivity for glucose (ability to select for glucose and not other substances), high stability, and high activity over a wide range of pH levels.
However, when GOx is directly combined with the bare electrode surface, not only GOx itself is easily exfoliated (stripped of some of its layers), but its biological activity and stability can also be affected. In addition, electron transfer efficiency between the GOx and the electrode surface is a key factor determining the sensitivity of the sensor.
So far, numerous attempts have been made at making the GOx enzyme more firmly attached to the electrode, thereby enhancing the direct electron transfer between the electroactive centers (sites of electron activity) and the electrode surface. One notable attempt involves the use of electrodes designed at the nanoscale to have structures on the electrode that provide larger surface areas and high electrocatalytic activity.
Unfortunately, these nanostructures increase the complexity of fabricating such electrochemical biosensors. Their construction also relies on the synthetic polymer Nafion as a scaffold, which creates a barrier for the charge transfer across the interface between the sensor and the fluid being tested.
The researchers have therefore gone in a completely different direction. The team aimed at improving glucose sensing performance by using colloidal quantum dots (CQDs) as the material for modifying the electrode. CQDs are "zero"-dimensional semiconductor nanoparticles. (They are not actually zero dimensions, but rather just extremely tiny diameters typically ranging from 2 to 20 nm). These possess an abundance of active sites—locations where chemical reactions can occur—and bind very stably to biological protein molecules.
Even better, due to their very tiny size, CQDs undergo quantum effects such as quantum tunneling, and the charge transfer at the CQD-protein interface can be regulated by the application of an external electric field. CQDs are also compatible with a range of different rigid and flexible substrate materials, making them more easily manufacturable.
Enhancing this effect, the researchers integrated gold nanospheres (AuNSs) into the structure of the sensor electrode. These are ultra-tiny spherical nanoparticles with diameters ranging from 10–200 nm. They are increasingly being used in biosensing applications due to their unique physical and chemical properties.
In particular, when used as a component in enzymatic electrochemical biosensors, AuNSs allow protein enzymes to retain their biological activity upon adhesion to surfaces and reduce the insulating effect of the shell of the protein for direct electron transfer. In a CGM, this greatly enhances the signal amplitude of the electrochemical biosensors.
The researchers constructed a proof-of-concept CGM employing CQDs—in this case made of lead sulfide—and the AuNSs-modified electrode. They found that the addition of the AuNSs in particular significantly improved the current signal detected by the electrochemical sensor, as had been hoped.
Combined, these alterations showed great potential in detecting glucose in different samples such as blood, sweat and other bodily fluid, and delivered a rapid (in less than 30 seconds) electrochemical biosensor, with a wide detection range and the sort of ultra-high sensitivity the team was seeking.
The researchers now aim to take their proof-of-concept CGM and make it manufacturable at commercial scale.
Provided by Tsinghua University Press.

 Previous page
Previous page Back to top
Back to top







